Direct submissions to PCI Ecology from bioRxiv.org are possible using the B2J service
Latest recommendations
| Id | Title * | Authors * | Abstract * | Picture * | Thematic fields * | Recommender | Reviewers | Submission date | |
|---|---|---|---|---|---|---|---|---|---|
27 Jan 2023
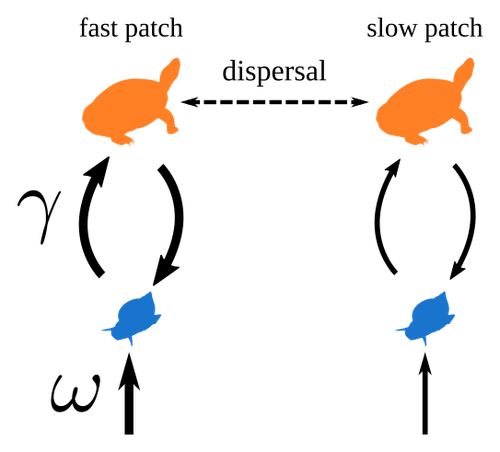
Spatial heterogeneity of interaction strength has contrasting effects on synchrony and stability in trophic metacommunitiesPierre Quévreux, Bart Haegeman and Michel Loreau https://hal.science/hal-03829838How does spatial heterogeneity affect stability of trophic metacommunities?Recommended by Werner Ulrich based on reviews by Phillip P.A. Staniczenko, Ludek Berec and Diogo Provete based on reviews by Phillip P.A. Staniczenko, Ludek Berec and Diogo Provete
The temporal or spatial variability in species population sizes and interaction strength of animal and plant communities has a strong impact on aggregate community properties (for instance biomass), community composition, and species richness (Kokkoris et al. 2002). Early work on spatial and temporal variability strongly indicated that asynchronous population and environmental fluctuations tend to stabilise community structures and diversity (e.g. Holt 1984, Tilman and Pacala 1993, McCann et al. 1998, Amarasekare and Nisbet 2001). Similarly, trophic networks might be stabilised by spatial heterogeneity (Hastings 1977) and an asymmetry of energy flows along food chains (Rooney et al. 2006). The interplay between temporal, spatial, and trophic heterogeneity within the meta-community concept has got much less interest. In the recent preprint in PCI Ecology, Quévreux et al. (2023) report that Spatial heterogeneity of interaction strength has contrasting effects on synchrony and stability in trophic metacommunities. These authors rightly notice that the interplay between trophic and spatial heterogeneity might induce contrasting effects depending on the internal dynamics of the system. Their contribution builds on prior work (Quévreux et al. 2021a, b) on perturbed trophic cascades. I found this paper particularly interesting because it is in the, now century-old, tradition to show that ecological things are not so easy. Since the 1930th, when Nicholson and Baily and others demonstrated that simple deterministic population models might generate stability and (pseudo-)chaos ecologists have realised that systems triggered by two or more independent processes might be intrinsically unpredictable and generate different outputs depending on the initial parameter settings. This resembles the three-body problem in physics. The present contribution of Quévreux et al. (2023) extends this knowledge to an example of a spatially explicit trophic model. Their main take-home message is that asymmetric energy flows in predator–prey relationships might have contrasting effects on the stability of metacommunities receiving localised perturbations. Stability is context dependent. Of course, the work is merely a theoretical exercise using a simplistic trophic model. It demands verification with field data. Nevertheless, we might expect even stronger unpredictability in more realistic multitrophic situations. Therefore, it should be seen as a proof of concept. Remember that increasing trophic connectance tends to destabilise food webs (May 1972). In this respect, I found the final outlook to bioconservation ambitious but substantiated. Biodiversity management needs a holistic approach focusing on all aspects of ecological functioning. I would add the need to see stability and biodiversity within an evolutionary perspective. References Amarasekare P, Nisbet RM (2001) Spatial Heterogeneity, Source‐Sink Dynamics, and the Local Coexistence of Competing Species. The American Naturalist, 158, 572–584. https://doi.org/10.1086/323586 Hastings A (1977) Spatial heterogeneity and the stability of predator-prey systems. Theoretical Population Biology, 12, 37–48. https://doi.org/10.1016/0040-5809(77)90034-X Holt RD (1984) Spatial Heterogeneity, Indirect Interactions, and the Coexistence of Prey Species. The American Naturalist, 124, 377–406. https://doi.org/10.1086/284280 Kokkoris GD, Jansen VAA, Loreau M, Troumbis AY (2002) Variability in interaction strength and implications for biodiversity. Journal of Animal Ecology, 71, 362–371. https://doi.org/10.1046/j.1365-2656.2002.00604.x May RM (1972) Will a Large Complex System be Stable? Nature, 238, 413–414. https://doi.org/10.1038/238413a0 McCann K, Hastings A, Huxel GR (1998) Weak trophic interactions and the balance of nature. Nature, 395, 794–798. https://doi.org/10.1038/27427 Quévreux P, Barbier M, Loreau M (2021) Synchrony and Perturbation Transmission in Trophic Metacommunities. The American Naturalist, 197, E188–E203. https://doi.org/10.1086/714131 Quévreux P, Pigeault R, Loreau M (2021) Predator avoidance and foraging for food shape synchrony and response to perturbations in trophic metacommunities. Journal of Theoretical Biology, 528, 110836. https://doi.org/10.1016/j.jtbi.2021.110836 Quévreux P, Haegeman B, Loreau M (2023) Spatial heterogeneity of interaction strength has contrasting effects on synchrony and stability in trophic metacommunities. hal-03829838, ver. 2 peer-reviewed and recommended by Peer Community in Ecology. https://hal.science/hal-03829838 Rooney N, McCann K, Gellner G, Moore JC (2006) Structural asymmetry and the stability of diverse food webs. Nature, 442, 265–269. https://doi.org/10.1038/nature04887 Tilman D, Pacala S (1993) The maintenance of species richness in plant communities. In: Ricklefs, R.E., Schluter, D. (eds) Species Diversity in Ecological Communities: Historical and Geographical Perspectives. University of Chicago Press, pp. 13–25. | Spatial heterogeneity of interaction strength has contrasting effects on synchrony and stability in trophic metacommunities | Pierre Quévreux, Bart Haegeman and Michel Loreau | <p> Spatial heterogeneity is a fundamental feature of ecosystems, and ecologists have identified it as a factor promoting the stability of population dynamics. In particular, differences in interaction strengths and resource supply between pa... |  | Dispersal & Migration, Food webs, Interaction networks, Spatial ecology, Metacommunities & Metapopulations, Theoretical ecology | Werner Ulrich | 2022-10-26 13:38:34 | View | |
24 Jan 2023
Four decades of phenology in an alpine amphibian: trends, stasis, and climatic driversOmar Lenzi, Kurt Grossenbacher, Silvia Zumbach, Beatrice Luescher, Sarah Althaus, Daniela Schmocker, Helmut Recher, Marco Thoma, Arpat Ozgul, Benedikt R. Schmidt https://doi.org/10.1101/2022.08.16.503739Alpine ecology and their dynamics under climate changeRecommended by Sergio Estay based on reviews by Nigel Yoccoz and 1 anonymous reviewerResearch about the effects of climate change on ecological communities has been abundant in the last decades. In particular, studies about the effects of climate change on mountain ecosystems have been key for understanding and communicating the consequences of this global phenomenon. Alpine regions show higher increases in warming in comparison to low-altitude ecosystems and this trend is likely to continue. This warming has caused reduced snowfall and/or changes in the duration of snow cover. For example, Notarnicola (2020) reported that 78% of the world’s mountain areas have experienced a snow cover decline since 2000. In the same vein, snow cover has decreased by 10% compared with snow coverage in the late 1960s (Walther et al., 2002) and snow cover duration has decreased at a rate of 5 days/decade (Choi et al., 2010). These changes have impacted the dynamics of high-altitude plant and animal populations. Some impacts are changes in the hibernation of animals, the length of the growing season for plants and the soil microbial composition (Chávez et al. 2021). Lenzi et al. (2023), give us an excellent study using long-term data on alpine amphibian populations. Authors show how climate change has impacted the reproductive phenology of Bufo bufo, especially the breeding season starts 30 days earlier than ~40 years ago. This earlier breeding is associated with the increasing temperatures and reduced snow cover in these alpine ecosystems. However, these changes did not occur in a linear trend but a marked acceleration was observed until mid-1990s with a later stabilization. Authors associated these nonlinear changes with complex interactions between the global trend of seasonal temperatures and site-specific conditions. Beyond the earlier breeding season, changes in phenology can have important impacts on the long-term viability of alpine populations. Complex interactions could involve positive and negative effects like harder environmental conditions for propagules, faster development of juveniles, or changes in predation pressure. This study opens new research opportunities and questions like the urgent assessment of the global impact of climate change on animal fitness. This study provides key information for the conservation of these populations. References Chávez RO, Briceño VF, Lastra JA, Harris-Pascal D, Estay SA (2021) Snow Cover and Snow Persistence Changes in the Mocho-Choshuenco Volcano (Southern Chile) Derived From 35 Years of Landsat Satellite Images. Frontiers in Ecology and Evolution, 9. https://doi.org/10.3389/fevo.2021.643850 Choi G, Robinson DA, Kang S (2010) Changing Northern Hemisphere Snow Seasons. Journal of Climate, 23, 5305–5310. https://doi.org/10.1175/2010JCLI3644.1 Lenzi O, Grossenbacher K, Zumbach S, Lüscher B, Althaus S, Schmocker D, Recher H, Thoma M, Ozgul A, Schmidt BR (2022) Four decades of phenology in an alpine amphibian: trends, stasis, and climatic drivers.bioRxiv, 2022.08.16.503739, ver. 3 peer-reviewed and recommended by Peer Community in Ecology. https://doi.org/10.1101/2022.08.16.503739 Notarnicola C (2020) Hotspots of snow cover changes in global mountain regions over 2000–2018. Remote Sensing of Environment, 243, 111781. https://doi.org/10.1016/j.rse.2020.111781 | Four decades of phenology in an alpine amphibian: trends, stasis, and climatic drivers | Omar Lenzi, Kurt Grossenbacher, Silvia Zumbach, Beatrice Luescher, Sarah Althaus, Daniela Schmocker, Helmut Recher, Marco Thoma, Arpat Ozgul, Benedikt R. Schmidt | <p style="text-align: justify;">Strong phenological shifts in response to changes in climatic conditions have been reported for many species, including amphibians, which are expected to breed earlier. Phenological shifts in breeding are observed i... |  | Climate change, Population ecology, Zoology | Sergio Estay | Anonymous, Nigel Yoccoz | 2022-08-18 08:25:21 | View |
28 Dec 2022

Deleterious effects of thermal and water stresses on life history and physiology: a case study on woodlouseCharlotte Depeux, Angele Branger, Theo Moulignier, Jérôme Moreau, Jean-Francois Lemaitre, Francois-Xavier Dechaume-Moncharmont, Tiffany Laverre, Hélène Paulhac, Jean-Michel Gaillard, Sophie Beltran-Bech https://doi.org/10.1101/2022.09.26.509512An experimental approach for understanding how terrestrial isopods respond to environmental stressorsRecommended by Aniruddha Belsare based on reviews by Aaron Yilmaz and Michael Morris based on reviews by Aaron Yilmaz and Michael Morris
In this article, the authors discuss the results of their study investigating the effects of heat stress and moisture stress on a terrestrial isopod Armadilldium vulgare, the common woodlouse [1]. Specifically, the authors have assessed how increased temperature or decreased moisture affects life history traits (such as growth, survival, and reproduction) as well as physiological traits (immune cell parameters and \( beta \)-galactosidase activity). This article quantitatively evaluates the effects of the two stressors on woodlouse. Terrestrial isopods like woodlouse are sensitive to thermal and moisture stress [2; 3] and are therefore good models to test hypotheses in global change biology and for monitoring ecosystem health. An important feature of this study is the combination of experimental, laboratory, and analytical techniques. Experiments were conducted under controlled conditions in the laboratory by modulating temperature and moisture, life history and physiological traits were measured/analyzed and then tested using models. Both stressors had negative impacts on survival and reproduction of woodlouse, and result in premature ageing. Although thermal stress did not affect survival, it slowed woodlouse growth. Moisture stress did not have a detectable effect on woodlouse growth but decreased survival and reproductive success. An important insight from this study is that effects of heat and moisture stressors on woodlouse are not necessarily linear, and experimental approaches can be used to better elucidate the mechanisms and understand how these organisms respond to environmental stress. This article is timely given the increasing attention on biological monitoring and ecosystem health. References: [1] Depeux C, Branger A, Moulignier T, Moreau J, Lemaître J-F, Dechaume-Moncharmont F-X, Laverre T, Pauhlac H, Gaillard J-M, Beltran-Bech S (2022) Deleterious effects of thermal and water stresses on life history and physiology: a case study on woodlouse. bioRxiv, 2022.09.26.509512., ver. 3 peer-reviewd and recommended by PCI Ecology. https://doi.org/10.1101/2022.09.26.509512 [2] Warburg MR, Linsenmair KE, Bercovitz K (1984) The effect of climate on the distribution and abundance of isopods. In: Sutton SL, Holdich DM, editors. The Biology of Terrestrial Isopods. Oxford: Clarendon Press. pp. 339–367. [3] Hassall M, Helden A, Goldson A, Grant A (2005) Ecotypic differentiation and phenotypic plasticity in reproductive traits of Armadillidium vulgare (Isopoda: Oniscidea). Oecologia 143: 51–60. https://doi.org/10.1007/s00442-004-1772-3 | Deleterious effects of thermal and water stresses on life history and physiology: a case study on woodlouse | Charlotte Depeux, Angele Branger, Theo Moulignier, Jérôme Moreau, Jean-Francois Lemaitre, Francois-Xavier Dechaume-Moncharmont, Tiffany Laverre, Hélène Paulhac, Jean-Michel Gaillard, Sophie Beltran-Bech | <p>We tested independently the influences of increasing temperature and decreasing moisture on life history and physiological traits in the arthropod <em>Armadillidium vulgare</em>. Both increasing temperature and decreasing moisture led individua... |  | Biodiversity, Evolutionary ecology, Experimental ecology, Life history, Physiology, Terrestrial ecology, Zoology | Aniruddha Belsare | 2022-09-28 13:13:47 | View | |
14 Dec 2022
The contrasted impacts of grasshoppers on soil microbial activities in function of primary production and herbivore dietSébastien Ibanez, Arnaud Foulquier, Charles Brun, Marie-Pascale Colace, Gabin Piton, Lionel Bernard, Christiane Gallet, Jean-Christophe Clément https://doi.org/10.1101/2022.07.04.497718Complex interactions between ecosystem productivity and herbivore diets lead to non-predicted effects on nutrient cyclingRecommended by Sébastien Barot based on reviews by Manuel Blouin and Tord Ranheim Sveen based on reviews by Manuel Blouin and Tord Ranheim Sveen
The authors present a study typical of the field of belowground-aboveground interactions [1]. This framework has been extremely fruitful since the beginning of 2000s [2]. It has also contributed to bridge the gap between soil ecology and the rest of ecology [3]. The study also pertains to the rich field on the impacts of herbivores on soil functioning [4]. The study more precisely tested during two years the effect on nutrient cycling of the interaction between the type of grassland (along a gradient of biomass productivity) and the diet of the community of insect herbivores (5 treatments manipulating the grasshopper community on 1 m2 plots, with a gradient from no grasshopper to grasshoppers either specialized on forbs or grasses). What seems extremely interesting is that the study is based on a rigorous hypothesis-testing approach. They compare the predictions of two frameworks: (1) The “productivity model” predicts that in productive ecosystems herbivores consume a high percentage of the net primary production thus accelerating nutrient cycling. (2) The “diet model” distinguishes herbivores consuming exploitative plants from those eating conservative plants. The former (later) type of herbivores favours conservative (exploitative) plants therefore decelerating (accelerating) nutrient cycling. Interestingly, the two frameworks have similar predictions (and symmetrically opposite predictions) in two cases out of four combinations between ecosystem productivities and types of diet (see Table 1). An other merit of the study is to combine in a rather comprehensive way all the necessary measurements to test these frameworks in combination: grasshopper diet, soil properties, characteristics of the soil microbial community, plant traits, vegetation survey and plant biomass. The results were in contradiction with the ‘‘diet model’’: microbial properties and nitrogen cycling did not depend on grasshopper diet. The productivity of the grasslands did impact nutrient cycling but not in the direction predicted by the “productivity model”: productive grasslands hosted exploitative plants that depleted N resources in the soil and microbes producing few extracellular enzymes, which led to a lower potential N mineralization and a deceleration of nutrient cycling. Because, the authors stuck to their original hypotheses (that were not confirmed), they were able to discuss in a very relevant way their results and to propose some interpretations, at least partially based on the time scales involved by the productivity and diet models. Beyond all the merits of this article, I think that two issues remain largely open in relation with the dynamics of the studied systems, and would deserve future research efforts. First, on the ‘‘short’’ term (up to several decades), can we predict how the communities of plants, soil microbes, and herbivores interact to drive the dynamics of the ecosystems? Second, at the evolutionary time scale, can we understand and predict the interactions between the evolution of plant, microbe and herbivore strategies and the consequences for the functioning of the grasslands? The two issues are difficult because of the multiple feedbacks involved. One way to go further would be to complement the empirical approach with models along existing research avenues [5, 6]. References [1] Ibanez S, Foulquier A, Brun C, Colace M-P, Piton G, Bernard L, Gallet C, Clément J-C (2022) The contrasted impacts of grasshoppers on soil microbial activities in function of primary production and herbivore diet. bioRxiv, 2022.07.04.497718, ver. 2 peer-reviewed and recommended by Peer Community in Ecology. https://doi.org/10.1101/2022.07.04.497718 [2] Hooper, D. U., Bignell, D. E., Brown, V. K., Brussaard, L., Dangerfield, J. M., Wall, D. H., Wardle, D. A., Coleman, D. C., Giller, K. E., Lavelle, P., Van der Putten, W. H., De Ruiter, P. C., et al. 2000. Interactions between aboveground and belowground biodiversity in terretrial ecosystems: patterns, mechanisms, and feedbacks. BioScience, 50, 1049-1061. https://doi.org/10.1641/0006-3568(2000)050[1049:IBAABB]2.0.CO;2 [3] Barot, S., Blouin, M., Fontaine, S., Jouquet, P., Lata, J.-C., and Mathieu, J. 2007. A tale of four stories: soil ecology, theory, evolution and the publication system. PLoS ONE, 2, e1248. https://doi.org/10.1371/journal.pone.0001248 [4] Bardgett, R. D., and Wardle, D. A. 2003. Herbivore-mediated linkages between aboveground and belowground communities. Ecology, 84, 2258-2268. https://doi.org/10.1890/02-0274 [5] Barot, S., Bornhofen, S., Loeuille, N., Perveen, N., Shahzad, T., and Fontaine, S. 2014. Nutrient enrichment and local competition influence the evolution of plant mineralization strategy, a modelling approach. J. Ecol., 102, 357-366. https://doi.org/10.1111/1365-2745.12200 [6] Schweitzer, J. A., Juric, I., van de Voorde, T. F. J., Clay, K., van der Putten, W. H., Bailey, J. K., and Fox, C. 2014. Are there evolutionary consequences of plant-soil feedbacks along soil gradients? Func. Ecol., 28, 55-64. https://doi.org/10.1111/1365-2435.12201
| The contrasted impacts of grasshoppers on soil microbial activities in function of primary production and herbivore diet | Sébastien Ibanez, Arnaud Foulquier, Charles Brun, Marie-Pascale Colace, Gabin Piton, Lionel Bernard, Christiane Gallet, Jean-Christophe Clément | <p style="text-align: justify;">Herbivory can have contrasted impacts on soil microbes and nutrient cycling, which has stimulated the development of conceptual frameworks exploring the links between below- and aboveground processes. The "productiv... | Ecosystem functioning, Herbivory, Soil ecology, Terrestrial ecology | Sébastien Barot | 2022-07-14 09:06:13 | View | ||
25 Nov 2022

Positive fitness effects help explain the broad range of Wolbachia prevalences in natural populationsPetteri Karisto, Anne Duplouy, Charlotte de Vries, Hanna Kokko https://doi.org/10.1101/2022.04.11.487824Population dynamics of Wolbachia symbionts playing Dr. Jekyll and Mr. HydeRecommended by Jorge Peña based on reviews by 3 anonymous reviewers"Good and evil are so close as to be chained together in the soul" Maternally inherited symbionts—microorganisms that pass from a female host to her progeny—have two main ways of increasing their own fitness. First, they can increase the fecundity or viability of infected females. This “positive fitness effects” strategy is the one commonly used by mutualistic symbionts, such as Buchnera aphidicola—the bacterial endosymbiont of the pea aphid, Acyrthosiphon pisum [4]. Second, maternally inherited symbionts can manipulate the reproduction of infected females in a way that enhances symbiont transmission at the expense of host fitness. A famous example of this “reproductive parasitism” strategy is the cytoplasmic incompatibility (CI) [3] induced by bacteria of the genus Wolbachia in their arthropod and nematode hosts. CI works as a toxin-antidote system, whereby the sperm of infected males is modified in a lethal way (toxin) that can only be reverted if the egg is also infected (antidote) [1]. As a result, CI imposes a kind of conditional sterility on their hosts: while infected females are compatible with both infected and uninfected males, uninfected females experience high offspring mortality if (and only if) they mate with infected males [7]. These two symbiont strategies (positive fitness effects versus reproductive parasitism) have been traditionally studied separately, both empirically and theoretically. However, it has become clear that the two strategies are not mutually exclusive, and that a reproductive parasite can simultaneously act as a mutualist—an infection type that has been dubbed “Jekyll and Hyde” [6], after the famous novella by Robert Louis Stevenson about kind scientist Dr. Jekyll and his evil alter ego, Mr. Hyde. In important previous work, Zug and Hammerstein [7] analyzed the consequences of positive fitness effects on the dynamics of different kind of infections, including “Jekyll and Hyde” infections characterized by CI and other reproductive parasitism strategies. Building on this and related modeling framework, Karisto et al. [2] re-investigate and expand on the interplay between positive fitness effects and reproductive parasitism in Wolbachia infections by focusing on CI in both diplodiploid and haplodiploid populations, and by paying particular attention to the mathematical assumption structure underlying their results. Karisto et al. begin by reviewing classic models of Wolbachia infections in diplodiploid populations that assume a “negative fitness effect” (modeled as a fertility penalty on infected females), characteristic of a pure strategy of reproductive parasitism. Together with the positive frequency-dependent effects due to CI (whereby the fitness benefits to symbionts infecting females increase with the proportion of infected males in the population) this results in population dynamics characterized by two stable equilibria (the Wolbachia-free state and an interior equilibrium with a high frequency of Wolbachia-carrying hosts) separated by an unstable interior equilibrium. Wolbachia can then spread once the initial frequency is above a threshold or an invasion barrier, but is prevented from fixing by a proportion of infections failing to be passed on to offspring. Karisto et al. show that, given the assumption of negative fitness effects, the stable interior equilibrium can never feature a Wolbachia prevalence below one-half. Moreover, they convincingly argue that a prevalence greater than but close to one-half is difficult to maintain in the presence of stochastic fluctuations, as in these cases the high-prevalence stable equilibrium would be too close to the unstable equilibrium signposting the invasion barrier. Karisto et al. then relax the assumption of negative fitness effects and allow for positive fitness effects (modeled as a fertility premium on infected females) in a diplodiploid population. They show that positive fitness effects may result in situations where the original invasion threshold is now absent, the bistable coexistence dynamics are transformed into purely co-existence dynamics, and Wolbachia symbionts can now invade when rare. Karisto et al. conclude that positive fitness effects provide a plausible and potentially testable explanation for the low frequencies of symbiont-carrying hosts that are sometimes observed in nature, which are difficult to reconcile with the assumption of negative fitness effects. Finally, Karisto et al. extend their analysis to haplodiploid host populations (where all fertilized eggs develop as females). Here, they investigate two types of cytoplasmic incompatibility: a female-killing effect, similar to the CI effect studied in diplodiploid populations (the “Leptopilina type” of Vavre et al. [5]) and a masculinization effect, where CI leads to the loss of paternal chromosomes and to the development of the offspring as a male (the “Nasonia type” of Vavre et al. [5]). The models are now two-sex, which precludes a complete analytical treatment, in particular regarding the stability of fixed points. Karisto et al. compensate by conducting large numerical analyses that support their claims. Importantly, all main conclusions regarding the interplay between positive fitness effects and reproductive parasitism continue to hold under haplodiploidy. All in all, the analysis and results by Karisto et al. suggest that it is not necessary to resort to classical (but depending on the situation, unlikely) mechanisms, such as ongoing invasion or source-sink dynamics, to explain arthropod populations featuring low-prevalent Wolbachia infections. Instead, low-frequency equilibria might be simply due to reproductive parasites conferring beneficial fitness effects, or Wolbachia symbionts playing Dr. Jekyll (positive fitness effects) and Mr. Hyde (cytoplasmatic incompatibility). References [1] Beckmann JF, Bonneau M, Chen H, Hochstrasser M, Poinsot D, Merçot H, Weill M, Sicard M, Charlat S (2019) The Toxin–Antidote Model of Cytoplasmic Incompatibility: Genetics and Evolutionary Implications. Trends in Genetics, 35, 175–185. https://doi.org/10.1016/j.tig.2018.12.004 [2] Karisto P, Duplouy A, Vries C de, Kokko H (2022) Positive fitness effects help explain the broad range of Wolbachia prevalences in natural populations. bioRxiv, 2022.04.11.487824, ver. 5 peer-reviewed and recommended by Peer Community in Ecology. https://doi.org/10.1101/2022.04.11.487824 [3] Laven H (1956) Cytoplasmic Inheritance in Culex. Nature, 177, 141–142. https://doi.org/10.1038/177141a0 [4] Perreau J, Zhang B, Maeda GP, Kirkpatrick M, Moran NA (2021) Strong within-host selection in a maternally inherited obligate symbiont: Buchnera and aphids. Proceedings of the National Academy of Sciences, 118, e2102467118. https://doi.org/10.1073/pnas.2102467118 [5] Vavre F, Fleury F, Varaldi J, Fouillet P, Bouletreau M (2000) Evidence for Female Mortality in Wolbachia-Mediated Cytoplasmic Incompatibility in Haplodiploid Insects: Epidemiologic and Evolutionary Consequences. Evolution, 54, 191–200. https://doi.org/10.1111/j.0014-3820.2000.tb00019.x [6] Zug R, Hammerstein P (2015) Bad guys turned nice? A critical assessment of Wolbachia mutualisms in arthropod hosts. Biological Reviews, 90, 89–111. https://doi.org/10.1111/brv.12098 [7] Zug R, Hammerstein P (2018) Evolution of reproductive parasites with direct fitness benefits. Heredity, 120, 266–281. https://doi.org/10.1038/s41437-017-0022-5 | Positive fitness effects help explain the broad range of Wolbachia prevalences in natural populations | Petteri Karisto, Anne Duplouy, Charlotte de Vries, Hanna Kokko | <p style="text-align: justify;">The bacterial endosymbiont <em>Wolbachia</em> is best known for its ability to modify its host’s reproduction by inducing cytoplasmic incompatibility (CI) to facilitate its own spread. Classical models predict eithe... |  | Host-parasite interactions, Population ecology | Jorge Peña | 2022-04-12 12:52:55 | View | |
14 Nov 2022

Estimating abundance of a recovering transboundary brown bear population with capture-recapture modelsCécile Vanpé, Blaise Piédallu, Pierre-Yves Quenette, Jérôme Sentilles, Guillaume Queney, Santiago Palazón, Ivan Afonso Jordana, Ramón Jato, Miguel Mari Elósegui Irurtia, Jordi Solà de la Torre, Olivier Gimenez https://doi.org/10.1101/2021.12.08.471719A new and efficient approach to estimate, from protocol and opportunistic data, the size and trends of populations: the case of the Pyrenean brown bearRecommended by Nicolas BECH based on reviews by Tim Coulson, Romain Pigeault and ?In this study, the authors report a new method for estimating the abundance of the Pyrenean brown bear population. Precisely, the methodology involved aims to apply Pollock's closed robust design (PCRD) capture-recapture models to estimate population abundance and trends over time. Overall, the results encourage the use of PCRD to study populations' demographic rates, while minimizing biases due to inter-individual heterogeneity in detection probabilities. Estimating the size and trends of animal population over time is essential for informing conservation status and management decision-making (Nichols & Williams 2006). This is particularly the case when the population is small, geographically scattered, and threatened. Although several methods can be used to estimate population abundance, they may be difficult to implement when individuals are rare, elusive, solitary, largely nocturnal, highly mobile, and/or occupy large home ranges in remote and/or rugged habitats. Moreover, in such standard methods,
However, these conditions are rarely met in real populations, such as wild mammals (e.g., Bellemain et al. 2005; Solbert et al. 2006), and therefore the risk of underestimating population size can rapidly increase because the assumption of perfect detection of all individuals in the population is violated. Focusing on the critically endangered Pyrenean brown bear that was close to extinction in the mid-1990s, the study by Vanpe et al. (2022), uses protocol and opportunistic data to describe a statistical modeling exercise to construct mark-recapture histories from 2008 to 2020. Among the data, the authors collected non-invasive samples such as a mixture of hair and scat samples used for genetic identification, as well as photographic trap data of recognized individuals. These data are then analyzed in RMark to provide detection and survival estimates. The final model (i.e. PCRD capture-recapture) is then used to provide Bayesian population estimates. Results show a five-fold increase in population size between 2008 and 2020, from 13 to 66 individuals. Thus, this study represents the first published annual abundance and temporal trend estimates of the Pyrenean brown bear population since 2008. Then, although the results emphasize that the PCRD estimates were broadly close to the MRS counts and had reasonably narrow associated 95% Credibility Intervals, they also highlight that the sampling effort is different according to individuals. Indeed, as expected, the detection of an individual depends on
Overall, the PCRD capture-recapture modelling approach, involved in this study, provides robust estimates of abundance and demographic rates of the Pyrenean brown bear population (with associated uncertainty) while minimizing and considering bias due to inter-individual heterogeneity in detection probabilities. The authors conclude that mark-recapture provides useful population estimates and urge wildlife ecologists and managers to use robust approaches, such as the RDPC capture-recapture model, when studying large mammal populations. This information is essential to inform management decisions and assess the conservation status of populations.
References Bellemain, E.V.A., Swenson, J.E., Tallmon, D., Brunberg, S. and Taberlet, P. (2005). Estimating population size of elusive animals with DNA from hunter-collected feces: four methods for brown bears. Cons. Biol. 19(1), 150-161. https://doi.org/10.1111/j.1523-1739.2005.00549.x Nichols, J.D. and Williams, B.K. (2006). Monitoring for conservation. Trends Ecol. Evol. 21(12), 668-673. https://doi.org/10.1016/j.tree.2006.08.007 Otis, D.L., Burnham, K.P., White, G.C. and Anderson, D.R. (1978). Statistical inference from capture data on closed animal populations. Wildlife Monographs (62), 3-135. Solberg, K.H., Bellemain, E., Drageset, O.M., Taberlet, P. and Swenson, J.E. (2006). An evaluation of field and non-invasive genetic methods to estimate brown bear (Ursus arctos) population size. Biol. Conserv. 128(2), 158-168. https://doi.org/10.1016/j.biocon.2005.09.025 Vanpé C, Piédallu B, Quenette P-Y, Sentilles J, Queney G, Palazón S, Jordana IA, Jato R, Elósegui Irurtia MM, de la Torre JS, and Gimenez O (2022) Estimating abundance of a recovering transboundary brown bear population with capture-recapture models. bioRxiv, 2021.12.08.471719, ver. 4 recommended and peer-reviewed by PCI Ecology. https://doi.org/10.1101/2021.12.08.471719 | Estimating abundance of a recovering transboundary brown bear population with capture-recapture models | Cécile Vanpé, Blaise Piédallu, Pierre-Yves Quenette, Jérôme Sentilles, Guillaume Queney, Santiago Palazón, Ivan Afonso Jordana, Ramón Jato, Miguel Mari Elósegui Irurtia, Jordi Solà de la Torre, Olivier Gimenez | <p>Estimating the size of small populations of large mammals can be achieved via censuses, or complete counts, of recognizable individuals detected over a time period: minimum detected (population) size (MDS). However, as a population grows larger... |  | Conservation biology, Demography, Population ecology | Nicolas BECH | 2022-01-20 10:49:59 | View | |
31 Oct 2022

Ten simple rules for working with high resolution remote sensing dataAdam L. Mahood, Maxwell Benjamin Joseph, Anna Spiers, Michael J. Koontz, Nayani Ilangakoon, Kylen Solvik, Nathan Quarderer, Joe McGlinchy, Victoria Scholl, Lise St. Denis, Chelsea Nagy, Anna Braswell, Matthew W. Rossi, Lauren Herwehe, Leah wasser, Megan Elizabeth Cattau, Virginia Iglesias, Fangfang Yao, Stefan Leyk, Jennifer Balch https://doi.org/10.31219/osf.io/kehqzPreventing misuse of high-resolution remote sensing dataRecommended by Eric Goberville based on reviews by Jane Wyngaard and 1 anonymous reviewer based on reviews by Jane Wyngaard and 1 anonymous reviewer
To observe, characterise, identify, understand, predict... This is the approach that researchers follow every day. This sequence is tirelessly repeated as the biological model, the targeted ecosystem and/or the experimental, environmental or modelling conditions change. This way of proceeding is essential in a world of rapid change in response to the frenetic pace of intensifying pressures and forcings that impact ecosystems. To better understand our Earth and the dynamics of its components, to map ecosystems and diversity patterns, and to identify changes, humanity had to demonstrate inventiveness and defy gravity. Gustave Hermite and Georges Besançon were the first to launch aloft balloons equipped with radio transmitters, making possible the transmission of meteorological data to observers in real time [1]. The development of aviation in the middle of the 20th century constituted a real leap forward for the frequent acquisition of aerial observations, leading to a significant improvement in weather forecasting models. The need for systematic collection of data as holistic as possible – an essential component for the observation of complex biological systems - has resulted in pushing the limits of technological prowess. The conquest of space and the concurrent development of satellite observations has largely contributed to the collection of a considerable mass of data, placing our Earth under the "macroscope" - a concept introduced to ecology in the early 1970s by Howard T. Odum (see [2]), and therefore allowing researchers to move towards a better understanding of ecological systems, deterministic and stochastic patterns … with the ultimate goal of improving management actions [2,3]. Satellite observations have been carried out for nearly five decades now [3] and have greatly contributed to a better qualitative and quantitative understanding of the functioning of our planet, its diversity, its climate... and to a better anticipation of possible future changes (e.g., [4-7]). This access to rich and complex sources of information, for which both spatial and temporal resolutions are increasingly fine, results in the implementation of increasingly complex computation-based analyses, in order to meet the need for a better understanding of ecological mechanisms and processes, and their possible changes. Steven Levitt stated that "Data is one of the most powerful mechanisms for telling stories". This is so true … Data should not be used as a guide to thinking and a critical judgment at each stage of the data exploitation process should not be neglected. This is what Mahood et al. [8] rightly remind us in their article "Ten simple rules for working with high-resolution remote sensing data" in which they provide the fundamentals to consider when working with data of this nature, a still underutilized resource in several topics, such as conservation biology [3]. In this unconventional article, presented in a pedagogical way, the authors remind different generations of readers how satellite data should be handled and processed. The authors aim to make the readers aware of the most frequent pitfalls encouraging them to use data adapted to their original question, the most suitable tools/methods/procedures, to avoid methodological overkill, and to ensure both ethical use of data and transparency in the research process. While access to high-resolution data is increasingly easy thanks to the implementation of dedicated platforms [4], and because of the development of easy-to-use processing software and pipelines, it is important to take the time to recall some of the essential rules and guidelines for managing them, from new users with little or no experience who will find in this article the recommendations, resources and advice necessary to start exploiting remote sensing data, to more experienced researchers. References [1] Jeannet P, Philipona R, and Richner H (2016). 8 Swiss upper-air balloon soundings since 1902. In: Willemse S, Furger M (2016) From weather observations to atmospheric and climate sciences in Switzerland: Celebrating 100 years of the Swiss Society for Meteorology. vdf Hochschulverlag AG. [2] Odum HT (2007) Environment, Power, and Society for the Twenty-First Century: The Hierarchy of Energy. Columbia University Press. [3] Boyle SA, Kennedy CM, Torres J, Colman K, Pérez-Estigarribia PE, Sancha NU de la (2014) High-Resolution Satellite Imagery Is an Important yet Underutilized Resource in Conservation Biology. PLOS ONE, 9, e86908. https://doi.org/10.1371/journal.pone.0086908 [4] Le Traon P-Y, Antoine D, Bentamy A, Bonekamp H, Breivik LA, Chapron B, Corlett G, Dibarboure G, DiGiacomo P, Donlon C, Faugère Y, Font J, Girard-Ardhuin F, Gohin F, Johannessen JA, Kamachi M, Lagerloef G, Lambin J, Larnicol G, Le Borgne P, Leuliette E, Lindstrom E, Martin MJ, Maturi E, Miller L, Mingsen L, Morrow R, Reul N, Rio MH, Roquet H, Santoleri R, Wilkin J (2015) Use of satellite observations for operational oceanography: recent achievements and future prospects. Journal of Operational Oceanography, 8, s12–s27. https://doi.org/10.1080/1755876X.2015.1022050 [5] Turner W, Rondinini C, Pettorelli N, Mora B, Leidner AK, Szantoi Z, Buchanan G, Dech S, Dwyer J, Herold M, Koh LP, Leimgruber P, Taubenboeck H, Wegmann M, Wikelski M, Woodcock C (2015) Free and open-access satellite data are key to biodiversity conservation. Biological Conservation, 182, 173–176. https://doi.org/10.1016/j.biocon.2014.11.048 [6] Melet A, Teatini P, Le Cozannet G, Jamet C, Conversi A, Benveniste J, Almar R (2020) Earth Observations for Monitoring Marine Coastal Hazards and Their Drivers. Surveys in Geophysics, 41, 1489–1534. https://doi.org/10.1007/s10712-020-09594-5 [7] Zhao Q, Yu L, Du Z, Peng D, Hao P, Zhang Y, Gong P (2022) An Overview of the Applications of Earth Observation Satellite Data: Impacts and Future Trends. Remote Sensing, 14, 1863. https://doi.org/10.3390/rs14081863 [8] Mahood AL, Joseph MB, Spiers A, Koontz MJ, Ilangakoon N, Solvik K, Quarderer N, McGlinchy J, Scholl V, Denis LS, Nagy C, Braswell A, Rossi MW, Herwehe L, Wasser L, Cattau ME, Iglesias V, Yao F, Leyk S, Balch J (2021) Ten simple rules for working with high resolution remote sensing data. OSFpreprints, ver. 6 peer-reviewed and recommended by Peer Community in Ecology. https://doi.org/10.31219/osf.io/kehqz | Ten simple rules for working with high resolution remote sensing data | Adam L. Mahood, Maxwell Benjamin Joseph, Anna Spiers, Michael J. Koontz, Nayani Ilangakoon, Kylen Solvik, Nathan Quarderer, Joe McGlinchy, Victoria Scholl, Lise St. Denis, Chelsea Nagy, Anna Braswell, Matthew W. Rossi, Lauren Herwehe, Leah wasser,... | <p>Researchers in Earth and environmental science can extract incredible value from high-resolution (sub-meter, sub-hourly or hyper-spectral) remote sensing data, but these data can be difficult to use. Correct, appropriate and competent use of su... |  | Biogeography, Landscape ecology, Macroecology, Spatial ecology, Metacommunities & Metapopulations, Terrestrial ecology | Eric Goberville | 2021-10-19 21:41:22 | View | |
02 Aug 2022

The effect of dominance rank on female reproductive success in social mammalsShivani, Elise Huchard, Dieter Lukas https://doi.org/10.32942/osf.io/rc8naWhen do dominant females have higher breeding success than subordinates? A meta-analysis across social mammals.Recommended by Matthieu Paquet based on reviews by 2 anonymous reviewers based on reviews by 2 anonymous reviewers
In this meta-analysis, Shivani et al. [1] investigate 1) whether dominance and reproductive success are generally associated across social mammals and 2) whether this relationship varies according to a) life history traits (e.g., stronger for species with large litter size), b) ecological conditions (e.g., stronger when resources are limited) and c) the social environment (e.g., stronger for cooperative breeders than for plural breeders). Generally, the results are consistent with their predictions, except there was no clear support for this relationship to be conditional on the ecological conditions. considered As I have previously recommended the preregistration of this study [2,3], I do not have much to add here, as such recommendation should not depend on the outcome of the study. What I would like to recommend is the whole scientific process performed by the authors, from preregistration sent for peer review, to preprint submission and post-study peer review. It is particularly recommendable to notice that this project was a Masters student project, which shows that it is possible and worthy to preregister studies, even for such rather short-term projects. I strongly congratulate the authors for choosing this process even for an early career short-term project. I think it should be made possible for short-term students to conduct a preregistration study as a research project, without having to present post-study results. I hope this study can encourage a shift in the way we sometimes evaluate students’ projects. I also recommend the readers to look into the whole pre- and post- study reviewing history of this manuscript and the associated preregistration, as it provides a better understanding of the process and a good example of the associated challenges and benefits [4]. It was a really enriching experience and I encourage others to submit and review preregistrations and registered reports!
References [1] Shivani, Huchard, E., Lukas, D. (2022). The effect of dominance rank on female reproductive success in social mammals. EcoEvoRxiv, rc8na, ver. 10 peer-reviewed and recommended by Peer Community in Ecology. https://doi.org/10.32942/osf.io/rc8na [2] Shivani, Huchard, E., Lukas, D. (2020). Preregistration - The effect of dominance rank on female reproductive success in social mammals In principle acceptance by PCI Ecology of the version 1.2 on 07 July 2020. https://dieterlukas.github.io/Preregistration_MetaAnalysis_RankSuccess.html [3] Paquet, M. (2020) Why are dominant females not always showing higher reproductive success? A preregistration of a meta-analysis on social mammals. Peer Community in Ecology, 100056. https://doi.org/10.24072/pci.ecology.100056 [4] Parker, T., Fraser, H., & Nakagawa, S. (2019). Making conservation science more reliable with preregistration and registered reports. Conservation Biology, 33(4), 747-750. https://doi.org/10.1111/cobi.13342 | The effect of dominance rank on female reproductive success in social mammals | Shivani, Elise Huchard, Dieter Lukas | <p>Life in social groups, while potentially providing social benefits, inevitably leads to conflict among group members. In many social mammals, such conflicts lead to the formation of dominance hierarchies, where high-ranking individuals consiste... |  | Behaviour & Ethology, Meta-analyses | Matthieu Paquet | 2021-10-13 18:26:42 | View | |
03 Jun 2022
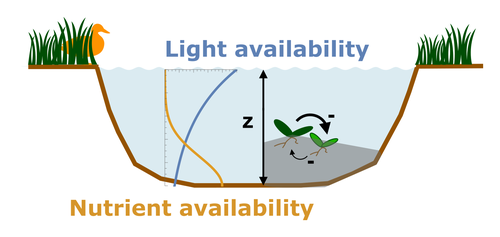
Evolutionary emergence of alternative stable states in shallow lakesAlice Ardichvili, Nicolas Loeuille, Vasilis Dakos https://doi.org/10.1101/2022.02.23.481597How to evolve an alternative stable stateRecommended by Tim Coulson based on reviews by Jean-François Arnoldi and 1 anonymous reviewer based on reviews by Jean-François Arnoldi and 1 anonymous reviewer
Alternative stable states describe ecosystems that can persist in more than one configuration. An ecosystem can shift between stable states following some form of perturbation. There has been much work on predicting when ecosystems will shift between stable states, but less work on why some ecosystems are able to exist in alternative stable states in the first place. The paper by Ardichvili, Loeuille, and Dakos (2022) addresses this question using a simple model of a shallow lake. Their model is based on a trade-off between access to light and nutrient availability in the water column, two essential resources for the macrophytes they model. They then identify conditions when the ancestral macrophyte will diversify resulting in macrophyte species living at new depths within the lake. The authors find a range of conditions where alternative stable states can evolve, but the range is narrow. Nonetheless, their model suggests that for alternative stable states to exist, one requirement is for there to be asymmetric competition between competing species, with one species being a better competitor on one limiting resource, with the other being a better competitor on a second limiting resource. These results are interesting and add to growing literature on how asymmetric competition can aid species coexistence. Asymmetric competition may be widespread in nature, with closely related species often being superior competitors on different resources. Incorporating asymmetric competition, and its evolution, into models does complicate theoretical investigations, but Ardichvili, Loeuille, and Dakos’ paper elegantly shows how substantial progress can be made with a model that is still (relatively) simple. References Ardichvili A, Loeuille N, Dakos V (2022) Evolutionary emergence of alternative stable states in shallow lakes. bioRxiv, 2022.02.23.481597, ver. 3 peer-reviewed and recommended by Peer Community in Ecology. https://doi.org/10.1101/2022.02.23.481597 | Evolutionary emergence of alternative stable states in shallow lakes | Alice Ardichvili, Nicolas Loeuille, Vasilis Dakos | <p style="text-align: justify;">Ecosystems under stress may respond abruptly and irreversibly through tipping points. Although much is explored on the mechanisms that affect tipping points and alternative stable states, little is known on how ecos... |  | Community ecology, Competition, Eco-evolutionary dynamics, Theoretical ecology | Tim Coulson | 2022-03-01 10:54:05 | View | |
31 May 2022

Sexual coercion in a natural mandrill populationNikolaos Smit, Alice Baniel, Berta Roura-Torres, Paul Amblard-Rambert, Marie J. E. Charpentier, Elise Huchard https://doi.org/10.1101/2022.02.07.479393Rare behaviours can have strong effects: evidence for sexual coercion in mandrillsRecommended by Matthieu Paquet based on reviews by Micaela Szykman Gunther and 1 anonymous reviewer based on reviews by Micaela Szykman Gunther and 1 anonymous reviewer
Sexual coercion can be defined as the use by a male of force, or threat of force, which increases the chances that a female will mate with him at a time when she is likely to be fertile, and/or decrease the chances that she will mate with other males, at some cost to the female (Smuts & Smuts 1993). It has been evidenced in a wide range of species and may play an important role in the evolution of sexual conflict and social systems. However, identifying sexual coercion in natural systems can be particularly challenging. Notably, while male behaviour may have immediate consequences on mating success (“harassment”), the mating benefits may be delayed in time (“intimidation”), and in such cases, evidencing coercion requires detailed temporal data at the individual level. Moreover, in some species male aggressive behaviours may be subtle or rare and hence hardly observed, yet still have important effects on female mating probability and fitness. Therefore, investigating the occurrence and consequences of sexual coercion in such species is particularly relevant but studying it in a statistically robust way is likely to require a considerable amount of time spent observing individuals. In this paper, Smit et al. (2022) test three clear predictions of the sexual coercion hypothesis in a natural population of Mandrills, where severe male aggression towards females is rare: (1) male aggression is more likely on sexually receptive females than on females in other reproductive states, (2) receptive females are more likely to be injured and (3) male aggression directed towards females is positively related to subsequent probability of copulation between those dyads. They also tested an alternative hypothesis, the “aggressive male phenotype” under which the correlation between male aggression towards females and subsequent mating could be statistically explained by male overall aggressivity. In agreement with the three predictions of the sexual coercion hypothesis, (1) male aggression was on average 5 times more likely, and (2) injuries twice as likely, to be observed on sexually receptive females than on females in other reproductive states and (3) copulation between males and sexually receptive females was twice more likely to be observed when aggression by this male was observed on the female before sexual receptivity. There was no support for the aggressive male hypothesis. The reviewers and I were highly positive about this study, notably regarding the way it is written and how the predictions are carefully and clearly stated, tested, interpreted, and discussed. This study is a good illustration of a case where some behaviours may not be common or obvious yet have strong effects and likely important consequences and thus be clearly worth studying. More generally, it shows once more the importance of detailed long-term studies at the individual level for our understanding of the ecology and evolution of wild populations. It is also a good illustration of the challenges faced, when comparing the likelihood of contrasting hypotheses means we need to alter sample sizes and/or the likelihood to observe at all some behaviours. For example, observing copulation within minutes after aggression (and therefore, showing statistical support for “harassment”) is inevitably less likely than observing copulations on the longer-term (and therefore showing statistical support for “intimidation”, when of course effort is put into recording such behavioural data on the long-term). Such challenges might partly explain some apparently intriguing results. For example, why are swollen females more aggressed by males if only aggression before the swollen period seems associated with more chances of mating? Here, the authors systematically provide effect sizes (and confidence intervals) and often describe the effects in an intuitive biological way (e.g., “Swollen females were, on average, about five times more likely to become injured”). This clearly helps the reader to not merely compare statistical significances but also the biological strengths of the estimated effects and the uncertainty around them. They also clearly acknowledge limits due to sample size when testing the harassment hypothesis, yet they provide precious information on the probability of observing mating (a rare behaviour) directly after aggression (already a rare behaviour!), that is, 3 times out of 38 aggressions observed between a male and a swollen female. Once again, this highlights how important it is to be able to pursue the enormous effort put so far into closely and continuously monitoring this wild population. Finally, this study raises exciting new questions, notably regarding to what extent females exhibit “counter-strategies” in response to sexual coercion, notably whether there is still scope for female mate choice under such conditions, and what are the fitness consequences of these dynamic conflicting sexual interactions. No doubt these questions will sooner than later be addressed by the authors, and I am looking forward to reading their upcoming work. References Smit N, Baniel A, Roura-Torres B, Amblard-Rambert P, Charpentier MJE, Huchard E (2022) Sexual coercion in a natural mandrill population. bioRxiv, 2022.02.07.479393, ver. 5 peer-reviewed and recommended by Peer Community in Ecology. https://doi.org/10.1101/2022.02.07.479393 Smuts BB, Smuts R w. (1993) Male Aggression and Sexual Coercion of Females in Nonhuman Primates and Other Mammals: Evidence and Theoretical Implications. In: Advances in the Study of Behavior (eds Slater PJB, Rosenblatt JS, Snowdon CT, Milinski M), pp. 1–63. Academic Press. https://doi.org/10.1016/S0065-3454(08)60404-0 | Sexual coercion in a natural mandrill population | Nikolaos Smit, Alice Baniel, Berta Roura-Torres, Paul Amblard-Rambert, Marie J. E. Charpentier, Elise Huchard | <p style="text-align: justify;">Increasing evidence indicates that sexual coercion is widespread. While some coercive strategies are conspicuous, such as forced copulation or sexual harassment, less is known about the ecology and evolution of inti... |  | Behaviour & Ethology | Matthieu Paquet | 2022-02-11 09:32:49 | View |
FOLLOW US
MANAGING BOARD
Julia Astegiano
Tim Coulson
Vasilis Dakos (Representative)
Anna Eklof
Dominique Gravel
François Massol
Ben Phillips
Cyrille Violle










