
BAROT Sébastien
- International Institute of Ecology and Environmental Sciences-Paris, IRD, Paris, France
- Agroecology, Coexistence, Ecosystem functioning, Experimental ecology, Soil ecology, Theoretical ecology, Tropical ecology
- recommender
Recommendations: 4
Reviews: 0
Recommendations: 4
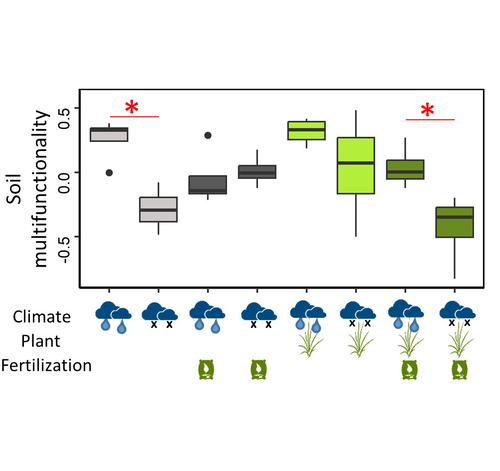
Mineral fertilization reduces the drought resistance of soil multifunctionality in a mountain grassland system through plant-soil interactions
Complex interactions between fertilization, drought and plants impact soil functioning
Recommended by Sébastien Barot based on reviews by 2 anonymous reviewersThe ingredients of this study are classic in soil ecology and in the study of belowground-aboveground interactions: the presence of plants, draught and mineral fertilization (for the experimental treatments) and microbial carbon, microbial nitrogen, microbial phosphorus, substrate-induced respiration, cumulative extracellular enzyme activity, nitrogen mineralization potential, nitrification potential, denitrification potential (as a result of the treatments). It is interesting and useful to have tested all the combinations of the three treatments and the height variables (also in the form of a soil multifunctionality index) in the same study and to have been able to express hypotheses on the underlying mechanisms of interaction.
A key result is that mineral fertilization can reduce the soil ability to withstand draughts in terms of soil multifunctionality. This effect would be due to the increase in plant growth associated with fertilization, which reduces the availability of soil resources. This subsequently affects microbial diversity and soil multifunctionality. This confirms that the interactions between plants and soil microorganisms are complex and relevant for understanding and predicting the impact of climate and fertilization on soil functioning and the sustainability of plant-soil systems.
Although the study is rather fundamental, it has been designed to be relevant to grassland management and points to very general mechanisms that are likely to be relevant to arable land management. It would therefore be useful to repeat this work for interactions between a crop and its soil. Finally, it would be crucial to test the impact of heavy fertilization in intensive cropping systems on the resistance and resilience of soil functions to climate variability and climate changes.
A slightly disturbing fact is that the underlying interactions are probably so complicated that it seems so far impossible to me to make predictions about the ranking of the height combinations of treatments on each soil variable. But this complexity is clearly inherent to ecology and, in particular, plant-soil interactions.
References
Gabin Piton, Arnaud Foulquier, Lionel Bernard, Aurelie Bonin, Thomas Pommier, Sandra Lavorel, Roberto Geremia, Jean Christophe Clement (2025) Mineral fertilization reduces the drought resistance of soil multifunctionality in a mountain grassland system through plant-soil interactions. bioRxiv, ver.2 peer-reviewed and recommended by PCI Ecology https://doi.org/10.1101/2024.09.19.613911
The contrasted impacts of grasshoppers on soil microbial activities in function of primary production and herbivore diet
Complex interactions between ecosystem productivity and herbivore diets lead to non-predicted effects on nutrient cycling
Recommended by Sébastien Barot based on reviews by Manuel Blouin and Tord Ranheim SveenThe authors present a study typical of the field of belowground-aboveground interactions [1]. This framework has been extremely fruitful since the beginning of 2000s [2]. It has also contributed to bridge the gap between soil ecology and the rest of ecology [3]. The study also pertains to the rich field on the impacts of herbivores on soil functioning [4].
The study more precisely tested during two years the effect on nutrient cycling of the interaction between the type of grassland (along a gradient of biomass productivity) and the diet of the community of insect herbivores (5 treatments manipulating the grasshopper community on 1 m2 plots, with a gradient from no grasshopper to grasshoppers either specialized on forbs or grasses). What seems extremely interesting is that the study is based on a rigorous hypothesis-testing approach. They compare the predictions of two frameworks: (1) The “productivity model” predicts that in productive ecosystems herbivores consume a high percentage of the net primary production thus accelerating nutrient cycling. (2) The “diet model” distinguishes herbivores consuming exploitative plants from those eating conservative plants. The former (later) type of herbivores favours conservative (exploitative) plants therefore decelerating (accelerating) nutrient cycling. Interestingly, the two frameworks have similar predictions (and symmetrically opposite predictions) in two cases out of four combinations between ecosystem productivities and types of diet (see Table 1). An other merit of the study is to combine in a rather comprehensive way all the necessary measurements to test these frameworks in combination: grasshopper diet, soil properties, characteristics of the soil microbial community, plant traits, vegetation survey and plant biomass.
The results were in contradiction with the ‘‘diet model’’: microbial properties and nitrogen cycling did not depend on grasshopper diet. The productivity of the grasslands did impact nutrient cycling but not in the direction predicted by the “productivity model”: productive grasslands hosted exploitative plants that depleted N resources in the soil and microbes producing few extracellular enzymes, which led to a lower potential N mineralization and a deceleration of nutrient cycling. Because, the authors stuck to their original hypotheses (that were not confirmed), they were able to discuss in a very relevant way their results and to propose some interpretations, at least partially based on the time scales involved by the productivity and diet models.
Beyond all the merits of this article, I think that two issues remain largely open in relation with the dynamics of the studied systems, and would deserve future research efforts. First, on the ‘‘short’’ term (up to several decades), can we predict how the communities of plants, soil microbes, and herbivores interact to drive the dynamics of the ecosystems? Second, at the evolutionary time scale, can we understand and predict the interactions between the evolution of plant, microbe and herbivore strategies and the consequences for the functioning of the grasslands? The two issues are difficult because of the multiple feedbacks involved. One way to go further would be to complement the empirical approach with models along existing research avenues [5, 6].
References
[1] Ibanez S, Foulquier A, Brun C, Colace M-P, Piton G, Bernard L, Gallet C, Clément J-C (2022) The contrasted impacts of grasshoppers on soil microbial activities in function of primary production and herbivore diet. bioRxiv, 2022.07.04.497718, ver. 2 peer-reviewed and recommended by Peer Community in Ecology. https://doi.org/10.1101/2022.07.04.497718
[2] Hooper, D. U., Bignell, D. E., Brown, V. K., Brussaard, L., Dangerfield, J. M., Wall, D. H., Wardle, D. A., Coleman, D. C., Giller, K. E., Lavelle, P., Van der Putten, W. H., De Ruiter, P. C., et al. 2000. Interactions between aboveground and belowground biodiversity in terretrial ecosystems: patterns, mechanisms, and feedbacks. BioScience, 50, 1049-1061. https://doi.org/10.1641/0006-3568(2000)050[1049:IBAABB]2.0.CO;2
[3] Barot, S., Blouin, M., Fontaine, S., Jouquet, P., Lata, J.-C., and Mathieu, J. 2007. A tale of four stories: soil ecology, theory, evolution and the publication system. PLoS ONE, 2, e1248. https://doi.org/10.1371/journal.pone.0001248
[4] Bardgett, R. D., and Wardle, D. A. 2003. Herbivore-mediated linkages between aboveground and belowground communities. Ecology, 84, 2258-2268. https://doi.org/10.1890/02-0274
[5] Barot, S., Bornhofen, S., Loeuille, N., Perveen, N., Shahzad, T., and Fontaine, S. 2014. Nutrient enrichment and local competition influence the evolution of plant mineralization strategy, a modelling approach. J. Ecol., 102, 357-366. https://doi.org/10.1111/1365-2745.12200
[6] Schweitzer, J. A., Juric, I., van de Voorde, T. F. J., Clay, K., van der Putten, W. H., Bailey, J. K., and Fox, C. 2014. Are there evolutionary consequences of plant-soil feedbacks along soil gradients? Func. Ecol., 28, 55-64. https://doi.org/10.1111/1365-2435.12201

Studies of NH4+ and NO3- uptake ability of subalpine plants and resource-use strategy identified by their functional traits
Nitrate or not nitrate. That is the question
Recommended by Sébastien Barot based on reviews by Vincent Maire and 1 anonymous reviewerThe article by Legay et al. [1] addresses two main issues: the links between belowground and aboveground plant traits and the links between plant strategies (as defined by these traits) and the capacity to absorb nitrate and ammonium. I recommend this work because these are important and current issues. The literature on plant traits is extremely rich and the existence of a leaf economic spectrum linked to a gradient between conservative and acquisitive plants is now extremely well established [2-3]. Many teams are now working on belowground traits and possible links with the aboveground gradients [4-5]. It seems indeed that there is a root economic spectrum but this spectrum is apparently less pronounced than the leaf economic spectrum. The existence of links between the two spectrums are still controversial and are likely not universal as suggested by discrepant results and after all a plant could have a conservative strategy aboveground and an acquisitive strategy belowground (or vice-versa) because, indeed, constraints are different belowground and aboveground (for example because in given ecosystem/vegetation type light may be abundant but not water or mineral nutrients). The various results obtained also suggest that we do not full understand the diversity of belowground strategies, what is at stake with these strategies, and the links with root characteristics.
Each time I give a conference on the work we are carrying out on African grasses that likely absorb ammonium preferentially because they inhibit nitrification [6-7], somebody asks me a question about the fact that plant essentially absorb nitrate because ammonium is toxic and nitrate more available in the soil. The present article confirms that this is not the case and that, though there are currently some teams working on the subject, we do not really know for the moment whether plants absorb nitrate or ammonium, in which proportion, how plastic this proportion is within individuals and within species. This subject seems to me crucial because it is linked to (1) the capacity of ecosystems to conserve nitrogen [8], because nitrate, much more than ammonium, goes out of ecosystems through leaching and denitrification, (2) to carbon cycling and plant energy budget because absorbing nitrate requires spending mucho more energy than absorbing ammonium because nitrate must be reduced before being incorporated in plant biomass, which is very energy costly. These two issues are naturally very relevant to develop efficient cropping systems in terms of carbon and nitrogen.
Interestingly, the present article, comparing three grass species in different sites, suggests that there is no trade-off between the absorption of nitrate and ammonium: more acquisitive individuals tend to absorb more ammonium and nitrate. This is contrary to hypotheses we made to predict the outcome of competition between plants absorbing nitrate and ammonium in different proportions [9] but should be tested in the future comparing many different types of plants. The results also suggest that more conservative plants absorb relatively more ammonium, which makes sense because this allows them to spare the energy necessary to reduce nitrate. This leads to the question of the effect of these strategies on nitrogen retention within the ecosystem. If nitrification is high (low), absorbing ammonium is not efficient and likely leads to high (low) nitrogen losses. This should be tested in the future. Moreover, the authors have measured the absorption of nitrate and ammonium through measurements at the root scale on cut roots. This should be complemented by measurements at the whole plant scale.
References
[1] Legay, N., Grassein, F., Arnoldi, C., Segura, R., Laîné, P., Lavorel, S. and Clément, J.-C. (2020). Studies of NH4+ and NO3- uptake ability of subalpine plants and resource-use strategy identified by their functional traits. bioRxiv, 372235, ver. 4 peer-reviewed and recommended by PCI Ecology. doi: 10.1101/372235
[2] Shipley, B., Lechowicz, M.J., Wright, I. & Reich, P.B. (2006) Fundamental trade-offs generating the worldwide leaf economics spectrum. Ecology, 87, 535-541. doi: 10.1890/05-1051
[3] Reich, P.B. (2014) The world-wide ‘fast-slow’ plant economics spectrum: a traits manifesto. J. Ecol., 102, 275-301. doi: 10.1111/1365-2745.12211
[4] Maire, V., Gross, N., Pontes, L.D.S., Picon-Cochard, C. & Soussana, J.F. (2009) Trade-off between root nitrogen acquisition and shoot nitrogen utilization across 13 co-occurring pasture grass species. Func. Ecol., 23, 668-679. doi: 10.1111/j.1365-2435.2009.01557.x
[5] Roumet, C., Birouste, M., Picon-Cochard, C., Ghestem, M., Osman, N., Vrignon-Brenas, S., Cao, K.F. & Stokes, A. (2016) Root structure-function relationships in 74 species: evidence of a root economics spectrum related to carbon economy. New. Phytol., 210, 815-826. doi: 10.1111/nph.13828
[6] Lata, J.-C., Degrange, V., Raynaud, X., Maron, P.-A., Lensi, R. & Abbadie, L. (2004) Grass populations control nitrification in savanna soils. Funct. Ecol., 18, 605-611. doi: 10.1111/j.0269-8463.2004.00880.x
[7] Srikanthasamy, T., Leloup, J., N’Dri, A.B., Barot, S., Gervaix, J., Koné, A.W., Koffi, K.F., Le Roux, X., Raynaud, X. & Lata, J.-C. (2018) Contrasting effects of grasses and trees on microbial N-cycling in an African humid savanna. Soil Biol. Biochem., 117, 153-163. doi: 10.1016/j.soilbio.2017.11.016
[8] Boudsocq, S., Lata, J.C., Mathieu, J., Abbadie, L. & Barot, S. (2009) Modelling approach to analyze the effects of nitrification inhibition on primary production. Func. Ecol., 23, 220-230. doi: 10.1111/j.1365-2435.2008.01476.x
[9] Boudsocq, S., Niboyet, A., Lata, J.-C., Raynaud, X., Loeuille, N., Mathieu, J., Blouin, M., Abbadie, L. & Barot, S. (2012) Plant preference for ammonium versus nitrate: a neglected determinant of ecosystem functioning? Am. Nat., 180, 60-69. doi: 10.1086/665997
Deer slow down litter decomposition by reducing litter quality in a temperate forest
Disentangling effects of large herbivores on litter decomposition
Recommended by Sébastien Barot based on reviews by 2 anonymous reviewersAboveground – belowground interactions is a fascinating field that has developed in ecology since about 20 years [1]. This field has been very fruitful as measured by the numerous articles published but also by the particular role it has played in the development of soil ecology. While soil ecology has for a long time developed partially independently from “general ecology” [2], the field of aboveground – belowground interactions has shown that all ecological interactions occurring within the soil are likely to impact plant growth and plant physiology because they have their roots within the soil. In turns, this should impact the aerial system of plants (higher or lower biomasses, changes in leaf quality…), which should cascade on the aboveground food web. Conversely, all ecological interactions occurring aboveground likely impact plant growth, which should cascade to their root systems, and thus to the soil functioning and the soil food web (through changes in the emission of exudates or inputs of dead roots…). Basically, plants are linking the belowground and aboveground worlds because, as terrestrial primary producers, they need to have (1) leaves to capture CO2 and exploit light and (2) roots to absorb water and mineral nutrients. The article I presently recommend [3] tackles this general issue through the prism of the impact of large herbivores on the decomposition of leaf litter.
This issue is a relatively old one [4, 5] but still deserves efforts because there have been relatively few studies on the subject and because the issue is relatively complex due to the diversity of mechanisms involved and the difficulty to disentangle them. I recommend this article because the authors have cleverly taken advantage of a ‘‘natural’’ long-term experiment, i.e. three islands with contrasted deer densities, to test whether these large mammals are able to impact leaf litter decomposition and whether they are able to do so through changes in litter quality (because they browse the vegetation) or through changes in soil characteristics (either physical or chemical characteristics or the composition of the decomposer community). They have found that deer decrease litter decomposition, mainly through a decrease in litter quality (increase in its C:N ratio). I particularly appreciate the combination of statistics achieved to test the different hypotheses and the fair and in-depth discussion of the results.
I have to confess that I have two small regrets with this work. First, all replications are implemented within the same three islands, so that it cannot be fully excluded that measured effects should not be attributed to any other possible difference between the three islands. I am fairly sure this is not the case (at least because the three islands have the same environments) but I hope that future studies or meta-analyses will be able analyse independent deer density treatments. Second, as a soil ecologist, I am eager to see results on the decomposer communities, both microorganisms and macrofauna, of the three islands.
References
[1] Hooper, D. U., Bignell, D. E., Brown, V. K., Brussard, L., Dangerfield, J. M., Wall, D. H. and Wolters, V. (2000). Interactions between Aboveground and Belowground Biodiversity in Terrestrial Ecosystems: Patterns, Mechanisms, and Feedbacks. BioScience, 50(12), 1049-1061. doi: 10.1641/0006-3568(2000)050[1049:ibaabb]2.0.co;2
[2] Barot, S., Blouin, M., Fontaine, S., Jouquet, P., Lata, J.-C., and Mathieu, J. (2007). A Tale of Four Stories: Soil Ecology, Theory, Evolution and the Publication System. PLOS ONE, 2(11), e1248. doi: 10.1371/journal.pone.0001248
[3] Chollet S., Maillard M., Schörghuber J., Grayston S. and Martin J.-L. (2019). Deer slow down litter decomposition by reducing litter quality in a temperate forest. bioRxiv, 690032, ver. 3 peer-reviewed and recommended by PCI Ecology. doi: 10.1101/690032
[4] Wardle, D. A., Barker, G. M., Yeates, G. W., Bonner, K. I., and Ghani, A. (2001). Introduced browsing mammals in New Zealand natural forests: aboveground and belowground consequences. Ecological Monographs, 71(4), 587-614. doi: 10.1890/0012-9615(2001)071[0587:ibminz]2.0.co;2
[5] Bardgett, R. D., and Wardle, D. A. (2003). Herbivore-mediated linkages between aboveground and belowground communities. Ecology, 84(9), 2258-2268. doi: 10.1890/02-0274