Latest recommendations

| Id | Title | Authors | Abstract▼ | Picture | Thematic fields | Recommender | Reviewers | Submission date | |
|---|---|---|---|---|---|---|---|---|---|
28 Mar 2024

Changes in length-at-first return of a sea trout (Salmo trutta) population in northern FranceQuentin Josset, Laurent Beaulaton, Atso Romakkaniemi, Marie Nevoux https://doi.org/10.1101/2023.11.21.568009Why are trout getting smaller?Recommended by Aleksandra Walczyńska based on reviews by Jan Kozlowski and 1 anonymous reviewerDecline in body size over time have been widely observed in fish (but see Solokas et al. 2023), and the ecological consequences of this pattern can be severe (e.g., Audzijonyte et al. 2013, Oke et al. 2020). Therefore, studying the interrelationships between life history traits to understand the causal mechanisms of this pattern is timely and valuable. This phenomenon was the subject of a study by Josset et al. (2024), in which the authors analysed data from 39 years of trout trapping in the Bresle River in France. The authors focused mainly on the length of trout on their first return from the sea. The most important results of the study were the decrease in fish length-at-first return and the change in the age structure of first-returning trout towards younger (and earlier) returning fish. It seems then that the smaller size of trout is caused by a shorter time spent in the sea rather than a change in a growth pattern, as length-at-age remained relatively constant, at least for those returning earlier. Fish returning after two years spent in the sea had a relatively smaller length-at-age. The authors suggest this may be due to local changes in conditions during fish's stay in the sea, although there is limited environmental data to confirm the causal effect. Another question is why there are fewer of these older fish. The authors point to possible increased mortality from disease and/or overfishing. These results may suggest that the situation may be getting worse, as another study finding was that “the more growth seasons an individual spent at sea, the greater was its length-at-first return.” The consequences may be the loss of the oldest and largest individuals, whose disproportionately high reproductive contribution to the population is only now understood (Barneche et al. 2018, Marshall and White 2019). Audzijonyte, A. et al. 2013. Ecological consequences of body size decline in harvested fish species: positive feedback loops in trophic interactions amplify human impact. Biol Lett 9, 20121103. https://doi.org/10.1098/rsbl.2012.1103 Oke, K. B. et al. 2020. Recent declines in salmon body size impact ecosystems and fisheries. Nature Communications, 11, 4155. https://doi.org/10.1038/s41467-020-17726-z Solokas, M. A. et al. 2023. Shrinking body size and climate warming: many freshwater salmonids do not follow the rule. Global Change Biology, 29, 2478-2492. https://doi.org/10.1111/gcb.16626 | Changes in length-at-first return of a sea trout (*Salmo trutta*) population in northern France | Quentin Josset, Laurent Beaulaton, Atso Romakkaniemi, Marie Nevoux | <p style="text-align: justify;">The resilience of sea trout populations is increasingly concerning, with evidence of major demographic changes in some populations. Based on trapping data and related scale collection, we analysed long-term changes ... |  | Biodiversity, Evolutionary ecology, Freshwater ecology, Life history, Marine ecology | Aleksandra Walczyńska | 2023-11-23 14:36:39 | View | |
27 Apr 2021

Joint species distributions reveal the combined effects of host plants, abiotic factors and species competition as drivers of species abundances in fruit fliesBenoit Facon, Abir Hafsi, Maud Charlery de la Masselière, Stéphane Robin, François Massol, Maxime Dubart, Julien Chiquet, Enric Frago, Frédéric Chiroleu, Pierre-François Duyck & Virginie Ravigné https://doi.org/10.1101/2020.12.07.414326Understanding the interplay between host-specificity, environmental conditions and competition through the sound application of Joint Species Distribution ModelsRecommended by Joaquín Hortal based on reviews by Joaquín Calatayud and Carsten DormannUnderstanding why and how species coexist in local communities is one of the central questions in ecology. There is general agreement that species distribution and coexistence are determined by a number of key mechanisms, including the environmental requirements of species, dispersal, evolutionary constraints, resource availability and selection, metapopulation dynamics, and biotic interactions (e.g. Soberón & Nakamura 2009; Colwell & Rangel 2009; Ricklefs 2015). These factors are however intricately intertwined in a scale-structured fashion (Hortal et al. 2010; D’Amen et al. 2017), making it particularly difficult to tease apart the effects of each one of them. This could be addressed by the novel field of Joint Species Distribution Modelling (JSDM; Okasvainen & Abrego 2020), as it allows assessing the effects of several sets of factors and the co-occurrence and/or covariation in abundances of potentially interacting species at the same time (Pollock et al. 2014; Ovaskainen et al. 2016; Dormann et al. 2018). However, the development of JSDM has been hampered by the general lack of good-quality detailed data on species co-occurrences and abundances (see Hortal et al. 2015). Facon et al. (2021) use a particularly large compilation of field surveys to study the abundance and co-occurrence of Tephritidae fruit flies in c. 400 orchards, gardens and natural areas throughout the island of Réunion. Further, they combine such information with lab data on their host-selection fundamental niche (i.e. in the absence of competitors), codifying traits of female choice and larval performances in 21 host species. They use Poisson Log-Normal models, a type of mixed model that allows one to jointly model the random effects associated with all species, and retrieve the covariations in abundance that are not explained by environmental conditions or differences in sampling effort. Then, they use a series of models to evaluate the effects on these matrices of ecological covariates (date, elevation, habitat, climate and host plant), species interactions (by comparing with a constrained residual variance-covariance matrix) and the species’ host-selection fundamental niches (through separate models for each fly species). The eight Tephritidae species inhabiting Réunion include both generalists and specialists in Solanaceae and Cucurbitaceae with a known history of interspecific competition. Facon et al. (2021) use a comprehensive JSDM approach to assess the effects of different factors separately and altogether. This allows them to identify large effects of plant hosts and the fundamental host-selection niche on species co-occurrence, but also to show that ecological covariates and weak –though not negligible– species interactions are necessary to account for all residual variance in the matrix of joint species abundances per site. Further, they also find evidence that the fitness per host measured in the lab has a strong influence on the abundances in each host plant in the field for specialist species, but not for generalists. Indeed, the stronger effects of competitive exclusion were found in pairs of Cucurbitaceae specialist species. However, these analyses fail to provide solid grounds to assess why generalists are rarely found in Cucurbitaceae and Solanaceae. Although they argue that this may be due to Connell’s (1980) ghost of competition past (past competition that led to current niche differentiation), further data on the evolutionary history of these fruit flies is needed to assess this hypothesis. Finding evidence for the effects of competitive interactions on species’ occurrences and spatial distributions is often difficult, perhaps because these effects occur over longer time scales than the ones usually studied by ecologists (Yackulic 2017). The work by Facon and colleagues shows that weak effects of competition can be detected also at the short ecological timescales that determine coexistence in local communities, under the virtuous combination of good-quality data and sound analytical designs that account for several aspects of species’ niches, their biotopes and their joint population responses. This adds a new dimension to the application of Hutchinson’s (1978) niche framework to understand the spatial dynamics of species and communities (see also Colwell & Rangel 2009), although further advances to incorporate dispersal-driven metacommunity dynamics (see, e.g., Ovaskainen et al. 2016; Leibold et al. 2017) are certainly needed. Nonetheless, this work shows the potential value of in-depth analyses of species coexistence based on combining good-quality field data with well-thought out JSDM applications. If many studies like this are conducted, it is likely that the uprising field of Joint Species Distribution Modelling will improve our understanding of the hierarchical relationships between the different factors affecting species coexistence in ecological communities in the near future.
References Colwell RK, Rangel TF (2009) Hutchinson’s duality: The once and future niche. Proceedings of the National Academy of Sciences, 106, 19651–19658. https://doi.org/10.1073/pnas.0901650106 Connell JH (1980) Diversity and the Coevolution of Competitors, or the Ghost of Competition Past. Oikos, 35, 131–138. https://doi.org/10.2307/3544421 D’Amen M, Rahbek C, Zimmermann NE, Guisan A (2017) Spatial predictions at the community level: from current approaches to future frameworks. Biological Reviews, 92, 169–187. https://doi.org/10.1111/brv.12222 Dormann CF, Bobrowski M, Dehling DM, Harris DJ, Hartig F, Lischke H, Moretti MD, Pagel J, Pinkert S, Schleuning M, Schmidt SI, Sheppard CS, Steinbauer MJ, Zeuss D, Kraan C (2018) Biotic interactions in species distribution modelling: 10 questions to guide interpretation and avoid false conclusions. Global Ecology and Biogeography, 27, 1004–1016. https://doi.org/10.1111/geb.12759 Facon B, Hafsi A, Masselière MC de la, Robin S, Massol F, Dubart M, Chiquet J, Frago E, Chiroleu F, Duyck P-F, Ravigné V (2021) Joint species distributions reveal the combined effects of host plants, abiotic factors and species competition as drivers of community structure in fruit flies. bioRxiv, 2020.12.07.414326. ver. 4 peer-reviewed and recommended by Peer community in Ecology. https://doi.org/10.1101/2020.12.07.414326 Hortal J, de Bello F, Diniz-Filho JAF, Lewinsohn TM, Lobo JM, Ladle RJ (2015) Seven Shortfalls that Beset Large-Scale Knowledge of Biodiversity. Annual Review of Ecology, Evolution, and Systematics, 46, 523–549. https://doi.org/10.1146/annurev-ecolsys-112414-054400 Hortal J, Roura‐Pascual N, Sanders NJ, Rahbek C (2010) Understanding (insect) species distributions across spatial scales. Ecography, 33, 51–53. https://doi.org/10.1111/j.1600-0587.2009.06428.x Hutchinson, G.E. (1978) An introduction to population biology. Yale University Press, New Haven, CT. Leibold MA, Chase JM, Ernest SKM (2017) Community assembly and the functioning of ecosystems: how metacommunity processes alter ecosystems attributes. Ecology, 98, 909–919. https://doi.org/10.1002/ecy.1697 Ovaskainen O, Abrego N (2020) Joint Species Distribution Modelling: With Applications in R. Cambridge University Press, Cambridge. https://doi.org/10.1017/9781108591720 Ovaskainen O, Roy DB, Fox R, Anderson BJ (2016) Uncovering hidden spatial structure in species communities with spatially explicit joint species distribution models. Methods in Ecology and Evolution, 7, 428–436. https://doi.org/10.1111/2041-210X.12502 Pollock LJ, Tingley R, Morris WK, Golding N, O’Hara RB, Parris KM, Vesk PA, McCarthy MA (2014) Understanding co-occurrence by modelling species simultaneously with a Joint Species Distribution Model (JSDM). Methods in Ecology and Evolution, 5, 397–406. https://doi.org/10.1111/2041-210X.12180 Ricklefs RE (2015) Intrinsic dynamics of the regional community. Ecology Letters, 18, 497–503. https://doi.org/10.1111/ele.12431 Soberón J, Nakamura M (2009) Niches and distributional areas: Concepts, methods, and assumptions. Proceedings of the National Academy of Sciences, 106, 19644–19650. https://doi.org/10.1073/pnas.0901637106 Yackulic CB (2017) Competitive exclusion over broad spatial extents is a slow process: evidence and implications for species distribution modeling. Ecography, 40, 305–313. https://doi.org/10.1111/ecog.02836 | Joint species distributions reveal the combined effects of host plants, abiotic factors and species competition as drivers of species abundances in fruit flies | Benoit Facon, Abir Hafsi, Maud Charlery de la Masselière, Stéphane Robin, François Massol, Maxime Dubart, Julien Chiquet, Enric Frago, Frédéric Chiroleu, Pierre-François Duyck & Virginie Ravigné | <p style="text-align: justify;">The relative importance of ecological factors and species interactions for phytophagous insect species distributions has long been a controversial issue. Using field abundances of eight sympatric Tephritid fruit fli... |  | Biodiversity, Coexistence, Community ecology, Competition, Herbivory, Interaction networks, Species distributions | Joaquín Hortal | Carsten Dormann, Joaquín Calatayud | 2020-12-08 06:44:25 | View |
01 Oct 2023
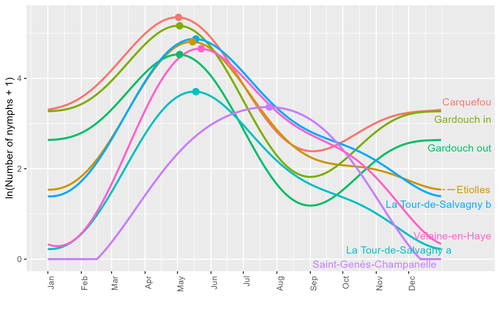
Seasonality of host-seeking Ixodes ricinus nymph abundance in relation to climateThierry Hoch, Aurélien Madouasse, Maude Jacquot, Phrutsamon Wongnak, Fréderic Beugnet, Laure Bournez, Jean-François Cosson, Frédéric Huard, Sara Moutailler, Olivier Plantard, Valérie Poux, Magalie René-Martellet, Muriel Vayssier-Taussat, Hélène Verheyden, Gwenaёl Vourc’h, Karine Chalvet-Monfray, Albert Agoulon https://doi.org/10.1101/2022.07.25.501416Assessing seasonality of tick abundance in different climatic regionsRecommended by Nigel Yoccoz based on reviews by 2 anonymous reviewers based on reviews by 2 anonymous reviewers
Tick-borne pathogens are considered as one of the major threats to public health – Lyme borreliosis being a well-known example of such disease. Global change – from climate change to changes in land use or invasive species – is playing a role in the increased risk associated with these pathogens. An important aspect of our knowledge of ticks and their associated pathogens is seasonality – one component being the phenology of within-year tick occurrences. This is important both in terms of health risk – e.g., when is the risk of encountering ticks high – and ecological understanding, as tick dynamics may depend on the weather as well as different hosts with their own dynamics and habitat use. Hoch et al. (2023) provide a detailed data set on the phenology of one species of tick, Ixodes ricinus, in 6 different locations of France. Whereas relatively cool sites showed a clear peak in spring-summer, warmer sites showed in addition relatively high occurrences in fall-winter, with a minimum in late summer-early fall. Such results add robust data to the existing evidence of the importance of local climatic patterns for explaining tick phenology. Recent analyses have shown that the phenology of Lyme borreliosis has been changing in northern Europe in the last 25 years, with seasonal peaks in cases occurring now 6 weeks earlier (Goren et al. 2023). The study by Hoch et al. (2023) is of too short duration to establish temporal changes in phenology (“only” 8 years, 2014-2021, see also Wongnak et al 2021 for some additional analyses; given the high year-to-year variability in weather, establishing phenological changes often require longer time series), and further work is needed to get better estimates of these changes and relate them to climate, land-use, and host density changes. Moreover, the phenology of ticks may also be related to species-specific tick phenology, and different tick species do not respond to current changes in identical ways (see for example differences between the two Ixodes species in Finland; Uusitalo et al. 2022). An efficient surveillance system requires therefore an adaptive monitoring design with regard to the tick species as well as the evolving causes of changes. References Goren, A., Viljugrein, H., Rivrud, I. M., Jore, S., Bakka, H., Vindenes, Y., & Mysterud, A. (2023). The emergence and shift in seasonality of Lyme borreliosis in Northern Europe. Proceedings of the Royal Society B: Biological Sciences, 290(1993), 20222420. https://doi.org/10.1098/rspb.2022.2420 Hoch, T., Madouasse, A., Jacquot, M., Wongnak, P., Beugnet, F., Bournez, L., . . . Agoulon, A. (2023). Seasonality of host-seeking Ixodes ricinus nymph abundance in relation to climate. bioRxiv, ver.4 peer-reviewed and recommended by Peer Community In Ecology. https://doi.org/10.1101/2022.07.25.501416 Uusitalo, R., Siljander, M., Lindén, A., Sormunen, J. J., Aalto, J., Hendrickx, G., . . . Vapalahti, O. (2022). Predicting habitat suitability for Ixodes ricinus and Ixodes persulcatus ticks in Finland. Parasites & Vectors, 15(1), 310. https://doi.org/10.1186/s13071-022-05410-8 Wongnak, P., Bord, S., Jacquot, M., Agoulon, A., Beugnet, F., Bournez, L., . . . Chalvet-Monfray, K. (2022). Meteorological and climatic variables predict the phenology of Ixodes ricinus nymph activity in France, accounting for habitat heterogeneity. Scientific Reports, 12(1), 7833. https://doi.org/10.1038/s41598-022-11479-z | Seasonality of host-seeking *Ixodes ricinus* nymph abundance in relation to climate | Thierry Hoch, Aurélien Madouasse, Maude Jacquot, Phrutsamon Wongnak, Fréderic Beugnet, Laure Bournez, Jean-François Cosson, Frédéric Huard, Sara Moutailler, Olivier Plantard, Valérie Poux, Magalie René-Martellet, Muriel Vayssier-Taussat, Hélène Ve... | <p style="text-align: justify;">There is growing concern about climate change and its impact on human health. Specifically, global warming could increase the probability of emerging infectious diseases, notably because of changes in the geographic... |  | Climate change, Population ecology, Statistical ecology | Nigel Yoccoz | 2022-10-14 18:43:56 | View | |
29 Sep 2023
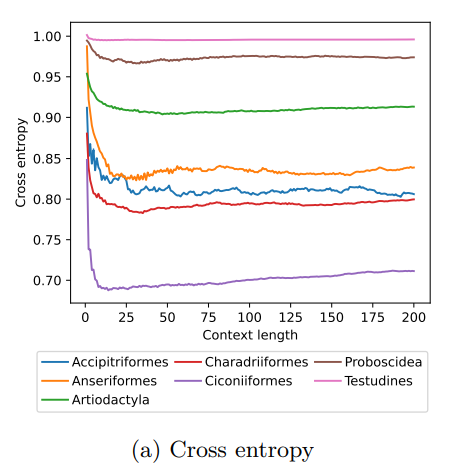
MoveFormer: a Transformer-based model for step-selection animal movement modellingOndřej Cífka, Simon Chamaillé-Jammes, Antoine Liutkus https://doi.org/10.1101/2023.03.05.531080A deep learning model to unlock secrets of animal movement and behaviourRecommended by Cédric Sueur based on reviews by Jacob Davidson and 1 anonymous reviewer based on reviews by Jacob Davidson and 1 anonymous reviewer
The study of animal movement is essential for understanding their behaviour and how ecological or global changes impact their routines [1]. Recent technological advancements have improved the collection of movement data [2], but limited statistical tools have hindered the analysis of such data [3–5]. Animal movement is influenced not only by environmental factors but also by internal knowledge and memory, which are challenging to observe directly [6,7]. Routine movement behaviours and the incorporation of memory into models remain understudied. Researchers have developed ‘MoveFormer’ [8], a deep learning-based model that predicts future movements based on past context, addressing these challenges and offering insights into the importance of different context lengths and information types. The model has been applied to a dataset of over 1,550 trajectories from various species, and the authors have made the MoveFormer source code available for further research. Inspired by the step-selection framework and efforts to quantify uncertainty in movement predictions, MoveFormer leverages deep learning, specifically the Transformer architecture, to encode trajectories and understand how past movements influence current and future ones – a critical question in movement ecology. The results indicate that integrating information from a few days to two or three weeks before the movement enhances predictions. The model also accounts for environmental predictors and offers insights into the factors influencing animal movements. Its potential impact extends to conservation, comparative analyses, and the generalisation of uncertainty-handling methods beyond ecology, with open-source code fostering collaboration and innovation in various scientific domains. Indeed, this method could be applied to analyse other kinds of movements, such as arm movements during tool use [9], pen movements, or eye movements during drawing [10], to better understand anticipation in actions and their intentionality. References 1. Méndez, V.; Campos, D.; Bartumeus, F. Stochastic Foundations in Movement Ecology: Anomalous Diffusion, Front Propagation and Random Searches; Springer Series in Synergetics; Springer: Berlin, Heidelberg, 2014; ISBN 978-3-642-39009-8. | MoveFormer: a Transformer-based model for step-selection animal movement modelling | Ondřej Cífka, Simon Chamaillé-Jammes, Antoine Liutkus | <p style="text-align: justify;">The movement of animals is a central component of their behavioural strategies. Statistical tools for movement data analysis, however, have long been limited, and in particular, unable to account for past movement i... |  | Behaviour & Ethology, Habitat selection | Cédric Sueur | 2023-03-22 16:32:14 | View | |
23 Oct 2023
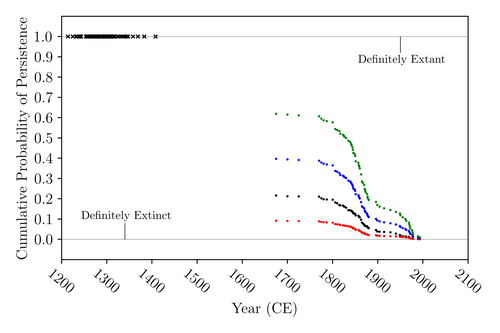
The Moa the Merrier: Resolving When the Dinornithiformes Went ExtinctFloe Foxon https://doi.org/10.1101/2023.08.07.552261Are Moas ancient Lazarus species?Recommended by Werner Ulrich based on reviews by Tim Coulson and Richard Holdaway based on reviews by Tim Coulson and Richard Holdaway
Ancient human colonisation often had catastrophic consequences for native fauna. The North American Megafauna went extinct shortly after humans entered the scene and Madagascar suffered twice, before 1500 CE and around 1700 CE after the Malayan and European colonisation. Maoris colonised New Zealand by about 1300 and a century later the giant Moa birds (Dinornithiformes) sharply declined. But did they went extinct or are they an ancient example of Lazarus species, species thought to be extinct but still alive? Scattered anecdotes of late sightings of living Moas even up to the 20th century seem to suggest the latter. The quest for later survival has also a criminal aspect. Who did it, the Maoris or the white colonisers in the late 18th century? The present work by Floe Foxon (2023) tries to settle this question. It uses a survival modelling approach and an assessment of the reliability of nearly 100 alleged sightings. The model favours the so-called overkill hypothesis, that Moas probably went extinct in the 15th century shortly after Maori colonisation. A small but still remarkable probability remained for survival up to 1770. Later sightings turned out to be highly unreliable. The paper is important as it does not rely on subjective discussions of late sightings but on a probabilistic modelling approach with sensitivity testing prior applied to marsupials. As common in probabilistic approaches, the study does not finally settle the case. A probability of as much as 20% remained for late survival after 1450 CE. This is not improbable as New Zealand was sufficiently unexplored in those days to harbour a few refuges for late survivors. However, in this respect, it is a bit unfortunate that at the end of the discussion, the paper cites Heuvelmans, the founder of cryptozoology, and it mentions the ivory-billed woodpecker, which has recently been redetected. No Moa remains were found after 1450. References Foxon F (2023) The Moa the Merrier: Resolving When the Dinornithiformes Went Extinct. bioRxiv, 2023.08.07.552261, ver. 2 peer-reviewed and recommended by Peer Community in Ecology. https://doi.org/10.1101/2023.08.07.552261 | The Moa the Merrier: Resolving When the Dinornithiformes Went Extinct | Floe Foxon | <p style="text-align: justify;">The Moa (Aves: Dinornithiformes) are an extinct group of the ratite clade from New Zealand. The overkill hypothesis asserts that the first New Zealand settlers hunted the Moa to extinction by 1450 CE, whereas the st... |  | Conservation biology, Human impact, Statistical ecology, Zoology | Werner Ulrich | Tim Coulson, Richard Holdaway | 2023-08-08 17:14:30 | View |
02 Aug 2021

Dynamics of Fucus serratus thallus photosynthesis and community primary production during emersion across seasons: canopy dampening and biochemical acclimationAline Migné, Gwendoline Duong, Dominique Menu, Dominique Davoult & François Gévaert https://hal.archives-ouvertes.fr/hal-03079617Towards a better understanding of the effects of self-shading on Fucus serratus populationsRecommended by Cédric Hubas based on reviews by Gwenael Abril, Francesca Rossi and 1 anonymous reviewer based on reviews by Gwenael Abril, Francesca Rossi and 1 anonymous reviewer
The importance of the vertical structure of vegetation cover for the functioning, management and conservation of ecosystems has received particular attention from ecologists in the last decades. Canopy architecture has many implications for light extinction coefficient, temperature variation reduction, self-shading which are all key parameters for the structuring and functioning of different ecosystems such as grasslands [1,2], forests [3,4], phytoplankton communities [5, 6], macroalgal populations [7] and even underwater animal forests such as octocoral communities [8]. This research topic, therefore, benefits from a large body of literature and the facilitative role of self-shadowing is no longer in question. However, it is always puzzling to note that some of the most common ecosystems turn out to be amongst the least known. This is precisely the case of the Fucus serratus communities which are widespread in Northeast Atlantic along the Atlantic coast of Europe from Svalbard to Portugal, as well as Northwest Atlantic & Gulf of St. Lawrence, easily accessible at low tide, but which have comparatively received less attention than more emblematic macro-algal communities such as Laminariales. The lack of attention paid to these most common Fucales is particularly critical as some species such as F. serratus are proving to be particularly vulnerable to environmental change, leading to a predicted northward retreat from its current southern boundary [9]. In the present study [10], the authors showed the importance of the vegetation cover in resisting tide-induced environmental stresses. The canopy of F. serratus mitigates stress levels experienced in the lower layers during emersion, while various acclimation strategies take over to maintain the photosynthetic apparatus in optimal conditions. They hereby highlight adaptation mechanisms to the extreme environment represented by the intertidal zone. These adaptation strategies were expected and similar mechanisms had been shown at the cellular level previously [11]. The earliest studies on the subject have shown that the structure of the bottom, the movement of water, and light availability all "influence the distribution of Fucaceae and disturb the regularity of their fine zonation, which itself is caused by the most important factor, desiccation", as Zaneveld states in his review [12]. He observed that the causes of the zonal distribution of marine algae are numerous, and identified several points of interest such as the relative period of emersion, the rapidity of desiccation, the loss of water, and the thickness of the cell walls. The present study thus highlights the existence of facilitative mechanisms associated with F. serratus canopy and nicely confirms previous work with in situ observations. It also highlights the importance of the vegetative cover in combating desiccation and introduces the dampening effect as a facilitating mechanism. The effect of the vegetation cover can sometimes even be felt beyond its immediate area of influence. A recent study shows that ground-level ozone is significantly reduced by the combined effects of canopy shading and turbulence [4]. Below the canopy, the light intensity becomes sufficiently low which inhibits ozone formation due to the decrease in the rates of hydroxyl radical formation and the rates of conversion of nitrogen dioxide to nitrogen oxide by photolysis. In addition, reductions in light levels associated with foliage promote ozone-destroying reactions between plant-emitted species, such as nitric oxide and/or alkenes, and ozone itself. The reduction in diffusivity slows the upward transport of surface emitted species, partially decoupling the area under the canopy from the rest of the atmosphere. By analogy with the work of Makar et al [4], and in the light of the results provided by the authors of this study, one may wonder whether the canopy dampening of F. serratus communities (and other common fucoids widely distributed on our coasts) might not also influence atmospheric chemistry, both at the Earth's surface and in the atmospheric boundary layer. The lack of accumulation of reactive oxygen species under the canopy found by the authors is consistent with this hypothesis and suggests that the damping effect of F. serratus may well have much wider consequences than expected. References [1] Jurik TW, Kliebenstein H (2000) Canopy Architecture, Light Extinction and Self-Shading of a Prairie Grass, Andropogon Gerardii. The American Midland Naturalist, 144, 51–65. http://www.jstor.org/stable/3083010 [2] Mitchley J, Willems JH (1995) Vertical canopy structure of Dutch chalk grasslands in relation to their management. Vegetatio, 117, 17–27. https://doi.org/10.1007/BF00033256 [3] Kane VR, Gillespie AR, McGaughey R, Lutz JA, Ceder K, Franklin JF (2008) Interpretation and topographic compensation of conifer canopy self-shadowing. Remote Sensing of Environment, 112, 3820–3832. https://doi.org/10.1016/j.rse.2008.06.001 [4] Makar PA, Staebler RM, Akingunola A, Zhang J, McLinden C, Kharol SK, Pabla B, Cheung P, Zheng Q (2017) The effects of forest canopy shading and turbulence on boundary layer ozone. Nature Communications, 8, 15243. https://doi.org/10.1038/ncomms15243 [5] Shigesada N, Okubo A (1981) Analysis of the self-shading effect on algal vertical distribution in natural waters. Journal of Mathematical Biology, 12, 311–326. https://doi.org/10.1007/BF00276919 [6] Barros MP, Pedersén M, Colepicolo P, Snoeijs P (2003) Self-shading protects phytoplankton communities against H2O2-induced oxidative damage. Aquatic Microbial Ecology, 30, 275–282. https://doi.org/10.3354/ame030275 [7] Ørberg SB, Krause-Jensen D, Mouritsen KN, Olesen B, Marbà N, Larsen MH, Blicher ME, Sejr MK (2018) Canopy-Forming Macroalgae Facilitate Recolonization of Sub-Arctic Intertidal Fauna and Reduce Temperature Extremes. Frontiers in Marine Science, 5. https://doi.org/10.3389/fmars.2018.00332 [8] Nelson H, Bramanti L (2020) From Trees to Octocorals: The Role of Self-Thinning and Shading in Underwater Animal Forests. In: Perspectives on the Marine Animal Forests of the World (eds Rossi S, Bramanti L), pp. 401–417. Springer International Publishing, Cham. https://doi.org/10.1007/978-3-030-57054-5_12 [9] Jueterbock A, Kollias S, Smolina I, Fernandes JMO, Coyer JA, Olsen JL, Hoarau G (2014) Thermal stress resistance of the brown alga Fucus serratus along the North-Atlantic coast: Acclimatization potential to climate change. Marine Genomics, 13, 27–36. https://doi.org/10.1016/j.margen.2013.12.008 [10] Migné A, Duong G, Menu D, Davoult D, Gévaert F (2021) Dynamics of Fucus serratus thallus photosynthesis and community primary production during emersion across seasons: canopy dampening and biochemical acclimation. HAL, hal-03079617, ver. 4 peer-reviewed and recommended by Peer community in Ecology. https://hal.archives-ouvertes.fr/hal-03079617 [11] Lichtenberg M, Kühl M (2015) Pronounced gradients of light, photosynthesis and O2 consumption in the tissue of the brown alga Fucus serratus. New Phytologist, 207, 559–569. https://doi.org/10.1111/nph.13396 [12] Zaneveld JS (1937) The Littoral Zonation of Some Fucaceae in Relation to Desiccation. Journal of Ecology, 25, 431–468. https://doi.org/10.2307/2256204 | Dynamics of Fucus serratus thallus photosynthesis and community primary production during emersion across seasons: canopy dampening and biochemical acclimation | Aline Migné, Gwendoline Duong, Dominique Menu, Dominique Davoult & François Gévaert | <p style="text-align: justify;">The brown alga <em>Fucus serratus</em> forms dense stands on the sheltered low intertidal rocky shores of the Northeast Atlantic coast. In the southern English Channel, these stands have proved to be highly producti... |  | Marine ecology | Cédric Hubas | 2021-01-05 16:24:02 | View | |
25 Nov 2022
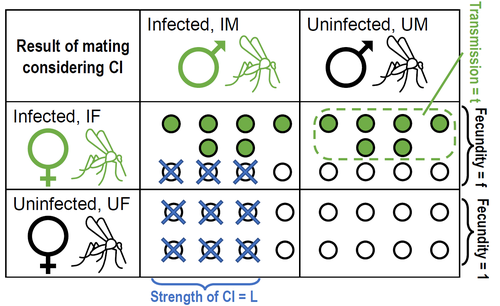
Positive fitness effects help explain the broad range of Wolbachia prevalences in natural populationsPetteri Karisto, Anne Duplouy, Charlotte de Vries, Hanna Kokko https://doi.org/10.1101/2022.04.11.487824Population dynamics of Wolbachia symbionts playing Dr. Jekyll and Mr. HydeRecommended by Jorge Peña based on reviews by 3 anonymous reviewers"Good and evil are so close as to be chained together in the soul" Maternally inherited symbionts—microorganisms that pass from a female host to her progeny—have two main ways of increasing their own fitness. First, they can increase the fecundity or viability of infected females. This “positive fitness effects” strategy is the one commonly used by mutualistic symbionts, such as Buchnera aphidicola—the bacterial endosymbiont of the pea aphid, Acyrthosiphon pisum [4]. Second, maternally inherited symbionts can manipulate the reproduction of infected females in a way that enhances symbiont transmission at the expense of host fitness. A famous example of this “reproductive parasitism” strategy is the cytoplasmic incompatibility (CI) [3] induced by bacteria of the genus Wolbachia in their arthropod and nematode hosts. CI works as a toxin-antidote system, whereby the sperm of infected males is modified in a lethal way (toxin) that can only be reverted if the egg is also infected (antidote) [1]. As a result, CI imposes a kind of conditional sterility on their hosts: while infected females are compatible with both infected and uninfected males, uninfected females experience high offspring mortality if (and only if) they mate with infected males [7]. These two symbiont strategies (positive fitness effects versus reproductive parasitism) have been traditionally studied separately, both empirically and theoretically. However, it has become clear that the two strategies are not mutually exclusive, and that a reproductive parasite can simultaneously act as a mutualist—an infection type that has been dubbed “Jekyll and Hyde” [6], after the famous novella by Robert Louis Stevenson about kind scientist Dr. Jekyll and his evil alter ego, Mr. Hyde. In important previous work, Zug and Hammerstein [7] analyzed the consequences of positive fitness effects on the dynamics of different kind of infections, including “Jekyll and Hyde” infections characterized by CI and other reproductive parasitism strategies. Building on this and related modeling framework, Karisto et al. [2] re-investigate and expand on the interplay between positive fitness effects and reproductive parasitism in Wolbachia infections by focusing on CI in both diplodiploid and haplodiploid populations, and by paying particular attention to the mathematical assumption structure underlying their results. Karisto et al. begin by reviewing classic models of Wolbachia infections in diplodiploid populations that assume a “negative fitness effect” (modeled as a fertility penalty on infected females), characteristic of a pure strategy of reproductive parasitism. Together with the positive frequency-dependent effects due to CI (whereby the fitness benefits to symbionts infecting females increase with the proportion of infected males in the population) this results in population dynamics characterized by two stable equilibria (the Wolbachia-free state and an interior equilibrium with a high frequency of Wolbachia-carrying hosts) separated by an unstable interior equilibrium. Wolbachia can then spread once the initial frequency is above a threshold or an invasion barrier, but is prevented from fixing by a proportion of infections failing to be passed on to offspring. Karisto et al. show that, given the assumption of negative fitness effects, the stable interior equilibrium can never feature a Wolbachia prevalence below one-half. Moreover, they convincingly argue that a prevalence greater than but close to one-half is difficult to maintain in the presence of stochastic fluctuations, as in these cases the high-prevalence stable equilibrium would be too close to the unstable equilibrium signposting the invasion barrier. Karisto et al. then relax the assumption of negative fitness effects and allow for positive fitness effects (modeled as a fertility premium on infected females) in a diplodiploid population. They show that positive fitness effects may result in situations where the original invasion threshold is now absent, the bistable coexistence dynamics are transformed into purely co-existence dynamics, and Wolbachia symbionts can now invade when rare. Karisto et al. conclude that positive fitness effects provide a plausible and potentially testable explanation for the low frequencies of symbiont-carrying hosts that are sometimes observed in nature, which are difficult to reconcile with the assumption of negative fitness effects. Finally, Karisto et al. extend their analysis to haplodiploid host populations (where all fertilized eggs develop as females). Here, they investigate two types of cytoplasmic incompatibility: a female-killing effect, similar to the CI effect studied in diplodiploid populations (the “Leptopilina type” of Vavre et al. [5]) and a masculinization effect, where CI leads to the loss of paternal chromosomes and to the development of the offspring as a male (the “Nasonia type” of Vavre et al. [5]). The models are now two-sex, which precludes a complete analytical treatment, in particular regarding the stability of fixed points. Karisto et al. compensate by conducting large numerical analyses that support their claims. Importantly, all main conclusions regarding the interplay between positive fitness effects and reproductive parasitism continue to hold under haplodiploidy. All in all, the analysis and results by Karisto et al. suggest that it is not necessary to resort to classical (but depending on the situation, unlikely) mechanisms, such as ongoing invasion or source-sink dynamics, to explain arthropod populations featuring low-prevalent Wolbachia infections. Instead, low-frequency equilibria might be simply due to reproductive parasites conferring beneficial fitness effects, or Wolbachia symbionts playing Dr. Jekyll (positive fitness effects) and Mr. Hyde (cytoplasmatic incompatibility). References [1] Beckmann JF, Bonneau M, Chen H, Hochstrasser M, Poinsot D, Merçot H, Weill M, Sicard M, Charlat S (2019) The Toxin–Antidote Model of Cytoplasmic Incompatibility: Genetics and Evolutionary Implications. Trends in Genetics, 35, 175–185. https://doi.org/10.1016/j.tig.2018.12.004 [2] Karisto P, Duplouy A, Vries C de, Kokko H (2022) Positive fitness effects help explain the broad range of Wolbachia prevalences in natural populations. bioRxiv, 2022.04.11.487824, ver. 5 peer-reviewed and recommended by Peer Community in Ecology. https://doi.org/10.1101/2022.04.11.487824 [3] Laven H (1956) Cytoplasmic Inheritance in Culex. Nature, 177, 141–142. https://doi.org/10.1038/177141a0 [4] Perreau J, Zhang B, Maeda GP, Kirkpatrick M, Moran NA (2021) Strong within-host selection in a maternally inherited obligate symbiont: Buchnera and aphids. Proceedings of the National Academy of Sciences, 118, e2102467118. https://doi.org/10.1073/pnas.2102467118 [5] Vavre F, Fleury F, Varaldi J, Fouillet P, Bouletreau M (2000) Evidence for Female Mortality in Wolbachia-Mediated Cytoplasmic Incompatibility in Haplodiploid Insects: Epidemiologic and Evolutionary Consequences. Evolution, 54, 191–200. https://doi.org/10.1111/j.0014-3820.2000.tb00019.x [6] Zug R, Hammerstein P (2015) Bad guys turned nice? A critical assessment of Wolbachia mutualisms in arthropod hosts. Biological Reviews, 90, 89–111. https://doi.org/10.1111/brv.12098 [7] Zug R, Hammerstein P (2018) Evolution of reproductive parasites with direct fitness benefits. Heredity, 120, 266–281. https://doi.org/10.1038/s41437-017-0022-5 | Positive fitness effects help explain the broad range of Wolbachia prevalences in natural populations | Petteri Karisto, Anne Duplouy, Charlotte de Vries, Hanna Kokko | <p style="text-align: justify;">The bacterial endosymbiont <em>Wolbachia</em> is best known for its ability to modify its host’s reproduction by inducing cytoplasmic incompatibility (CI) to facilitate its own spread. Classical models predict eithe... |  | Host-parasite interactions, Population ecology | Jorge Peña | 2022-04-12 12:52:55 | View | |
24 Jan 2023
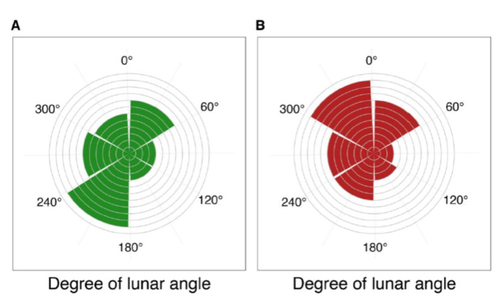
Four decades of phenology in an alpine amphibian: trends, stasis, and climatic driversOmar Lenzi, Kurt Grossenbacher, Silvia Zumbach, Beatrice Luescher, Sarah Althaus, Daniela Schmocker, Helmut Recher, Marco Thoma, Arpat Ozgul, Benedikt R. Schmidt https://doi.org/10.1101/2022.08.16.503739Alpine ecology and their dynamics under climate changeRecommended by Sergio Estay based on reviews by Nigel Yoccoz and 1 anonymous reviewerResearch about the effects of climate change on ecological communities has been abundant in the last decades. In particular, studies about the effects of climate change on mountain ecosystems have been key for understanding and communicating the consequences of this global phenomenon. Alpine regions show higher increases in warming in comparison to low-altitude ecosystems and this trend is likely to continue. This warming has caused reduced snowfall and/or changes in the duration of snow cover. For example, Notarnicola (2020) reported that 78% of the world’s mountain areas have experienced a snow cover decline since 2000. In the same vein, snow cover has decreased by 10% compared with snow coverage in the late 1960s (Walther et al., 2002) and snow cover duration has decreased at a rate of 5 days/decade (Choi et al., 2010). These changes have impacted the dynamics of high-altitude plant and animal populations. Some impacts are changes in the hibernation of animals, the length of the growing season for plants and the soil microbial composition (Chávez et al. 2021). Lenzi et al. (2023), give us an excellent study using long-term data on alpine amphibian populations. Authors show how climate change has impacted the reproductive phenology of Bufo bufo, especially the breeding season starts 30 days earlier than ~40 years ago. This earlier breeding is associated with the increasing temperatures and reduced snow cover in these alpine ecosystems. However, these changes did not occur in a linear trend but a marked acceleration was observed until mid-1990s with a later stabilization. Authors associated these nonlinear changes with complex interactions between the global trend of seasonal temperatures and site-specific conditions. Beyond the earlier breeding season, changes in phenology can have important impacts on the long-term viability of alpine populations. Complex interactions could involve positive and negative effects like harder environmental conditions for propagules, faster development of juveniles, or changes in predation pressure. This study opens new research opportunities and questions like the urgent assessment of the global impact of climate change on animal fitness. This study provides key information for the conservation of these populations. References Chávez RO, Briceño VF, Lastra JA, Harris-Pascal D, Estay SA (2021) Snow Cover and Snow Persistence Changes in the Mocho-Choshuenco Volcano (Southern Chile) Derived From 35 Years of Landsat Satellite Images. Frontiers in Ecology and Evolution, 9. https://doi.org/10.3389/fevo.2021.643850 Choi G, Robinson DA, Kang S (2010) Changing Northern Hemisphere Snow Seasons. Journal of Climate, 23, 5305–5310. https://doi.org/10.1175/2010JCLI3644.1 Lenzi O, Grossenbacher K, Zumbach S, Lüscher B, Althaus S, Schmocker D, Recher H, Thoma M, Ozgul A, Schmidt BR (2022) Four decades of phenology in an alpine amphibian: trends, stasis, and climatic drivers.bioRxiv, 2022.08.16.503739, ver. 3 peer-reviewed and recommended by Peer Community in Ecology. https://doi.org/10.1101/2022.08.16.503739 Notarnicola C (2020) Hotspots of snow cover changes in global mountain regions over 2000–2018. Remote Sensing of Environment, 243, 111781. https://doi.org/10.1016/j.rse.2020.111781 | Four decades of phenology in an alpine amphibian: trends, stasis, and climatic drivers | Omar Lenzi, Kurt Grossenbacher, Silvia Zumbach, Beatrice Luescher, Sarah Althaus, Daniela Schmocker, Helmut Recher, Marco Thoma, Arpat Ozgul, Benedikt R. Schmidt | <p style="text-align: justify;">Strong phenological shifts in response to changes in climatic conditions have been reported for many species, including amphibians, which are expected to breed earlier. Phenological shifts in breeding are observed i... |  | Climate change, Population ecology, Zoology | Sergio Estay | Anonymous, Nigel Yoccoz | 2022-08-18 08:25:21 | View |
21 Nov 2023
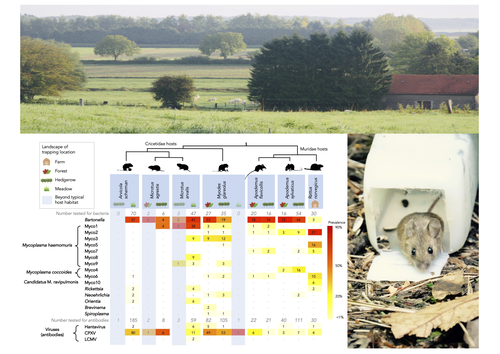
Pathogen community composition and co-infection patterns in a wild community of rodentsJessica Lee Abbate, Maxime Galan, Maria Razzauti, Tarja Sironen, Liina Voutilainen, Heikki Henttonen, Patrick Gasqui, Jean-François Cosson, Nathalie Charbonnel https://doi.org/10.1101/2020.02.09.940494Reservoirs of pestilence: what pathogen and rodent community analyses can tell us about transmission riskRecommended by Francois Massol based on reviews by Adrian Diaz, Romain Pigeault and 1 anonymous reviewer based on reviews by Adrian Diaz, Romain Pigeault and 1 anonymous reviewer
Rodents are well known as one of the main animal groups responsible for human-transmitted pathogens. As such, it seems logical to try and survey what kinds of pathogenic microbes might be harboured by wild rodents, in order to establish some baseline surveillance and prevent future zoonotic outbreaks (Bernstein et al., 2022). This is exactly what Abbate et al. (2023) endeavoured and their findings are intimidating. Based on quite a large sampling effort, they collected more than 700 rodents of seven species around two villages in northeastern France. They looked for molecular markers indicative of viral and bacterial infections and proceeded to analyze their pathogen communities using multivariate techniques. Variation in the prevalence of the different pathogens was found among host species, with e.g. signs of CPXV more prevalent in Cricetidae while some Mycoplasma strains were more prevalent in Muridae. Co-circulation of pathogens was found in all species, with some evidencing signs of up to 12 different pathogen taxa. The diversity of co-circulating pathogens was markedly different between host species and higher in adult hosts, but not affected by sex. The dataset also evinced some slight differences between habitats, with meadows harbouring a little more diversity of rodent pathogens than forests. Less intuitively, some pathogen associations seemed quite repeatable, such as the positive association of Bartonella spp. with CPXV in the montane water vole. The study allowed the authors to test several associations already described in the literature, including associations between different hemotropic Mycoplasma species. I strongly invite colleagues interested in zoonoses, emerging pandemics and more generally One Health to read the paper of Abbate et al. (2023) and try to replicate them across the world. To prevent the next sanitary crises, monitoring rodents, and more generally vertebrates, population demographics is a necessary and enlightening step (Johnson et al., 2020), but insufficient. Following the lead of colleagues working on rodent ectoparasites (Krasnov et al., 2014), we need more surveys like the one described by Abbate et al. (2023) to understand the importance of the dilution effect in the prevalence and transmission of microbial pathogens (Andreazzi et al., 2023) and the formation of epidemics. We also need other similar studies to assess the potential of different rodent species to carry pathogens more or less capable of infecting other mammalian species (Morand et al., 2015), in other places in the world. References Abbate, J. L., Galan, M., Razzauti, M., Sironen, T., Voutilainen, L., Henttonen, H., Gasqui, P., Cosson, J.-F. & Charbonnel, N. (2023) Pathogen community composition and co-infection patterns in a wild community of rodents. BioRxiv, ver.4 peer-reviewed and recommended by Peer Community in Ecology. https://doi.org/10.1101/2020.02.09.940494 Andreazzi, C. S., Martinez-Vaquero, L. A., Winck, G. R., Cardoso, T. S., Teixeira, B. R., Xavier, S. C. C., Gentile, R., Jansen, A. M. & D'Andrea, P. S. (2023) Vegetation cover and biodiversity reduce parasite infection in wild hosts across ecological levels and scales. Ecography, 2023, e06579. | Pathogen community composition and co-infection patterns in a wild community of rodents | Jessica Lee Abbate, Maxime Galan, Maria Razzauti, Tarja Sironen, Liina Voutilainen, Heikki Henttonen, Patrick Gasqui, Jean-François Cosson, Nathalie Charbonnel | <p style="text-align: justify;">Rodents are major reservoirs of pathogens that can cause disease in humans and livestock. It is therefore important to know what pathogens naturally circulate in rodent populations, and to understand the factors tha... |  | Biodiversity, Coexistence, Community ecology, Eco-immunology & Immunity, Epidemiology, Host-parasite interactions, Population ecology, Species distributions | Francois Massol | 2020-02-11 12:42:28 | View | |
26 May 2023
Using repeatability of performance within and across contexts to validate measures of behavioral flexibilityMcCune KB, Blaisdell AP, Johnson-Ulrich Z, Lukas D, MacPherson M, Seitz BM, Sevchik A, Logan CJ https://doi.org/10.32942/X2R59KDo reversal learning methods measure behavioral flexibility?Recommended by Aurélie Coulon based on reviews by Maxime Dahirel and Aparajitha Ramesh based on reviews by Maxime Dahirel and Aparajitha Ramesh
Assessing the reliability of the methods we use in actually measuring the intended trait should be one of our first priorities when designing a study – especially when the trait in question is not directly observable and is measured through a proxy. This is the case for cognitive traits, which are often quantified through measures of behavioral performance. Behavioral flexibility is of particular interest in the context of great environmental changes that a lot of populations have to experiment. This type of behavioral performance is often measured through reversal learning experiments (Bond 2007). In these experiments, individuals first learn a preference, for example for an object of a certain type of form or color, associated with a reward such as food. The characteristics of the rewarded object then change, and the individuals hence have to learn these new characteristics (to get the reward). The time needed by the individual to make this change in preference has been considered a measure of behavioral flexibility. Although reversal learning experiments have been widely used, their construct validity to assess behavioral flexibility has not been thoroughly tested. This was the aim of McCune and collaborators' (2023) study, through the test of the repeatability of individual performance within and across contexts of reversal learning, in the great-tailed grackle. This manuscript presents a post-study of the preregistered study* (Logan et al. 2019) that was peer-reviewed and received an In Principle Recommendation for PCI Ecology (Coulon 2019; the initial preregistration was split into 3 post-studies).
The first hypothesis was tested by measuring the repeatability of the time needed by individuals to switch color preference in a color reversal learning task (colored tubes), over serial sessions of this task. The second one was tested by measuring the time needed by individuals to switch solutions, within 3 different contexts: (1) colored tubes, (2) plastic and (3) wooden multi-access boxes involving several ways to access food. Despite limited sample sizes, the results of these experiments suggest that there is both temporal and contextual repeatability of behavioral flexibility performance of great-tailed grackles, as measured by reversal learning experiments. Those results are a first indication of the construct validity of reversal learning experiments to assess behavioral flexibility. As highlighted by McCune and collaborators, it is now necessary to assess the discriminant validity of these experiments, i.e. checking that a different performance is obtained with tasks (experiments) that are supposed to measure different cognitive abilities. Coulon, A. (2019) Can context changes improve behavioral flexibility? Towards a better understanding of species adaptability to environmental changes. Peer Community in Ecology, 100019. https://doi.org/10.24072/pci.ecology.100019 Logan, CJ, Lukas D, Bergeron L, Folsom M, & McCune, K. (2019). Is behavioral flexibility related to foraging and social behavior in a rapidly expanding species? In Principle Acceptance by PCI Ecology of the Version on 6 Aug 2019. http://corinalogan.com/Preregistrations/g_flexmanip.html McCune KB, Blaisdell AP, Johnson-Ulrich Z, Lukas D, MacPherson M, Seitz BM, Sevchik A, Logan CJ (2023) Using repeatability of performance within and across contexts to validate measures of behavioral flexibility. EcoEvoRxiv, ver. 5 peer-reviewed and recommended by Peer Community in Ecology. https://doi.org/10.32942/X2R59K | Using repeatability of performance within and across contexts to validate measures of behavioral flexibility | McCune KB, Blaisdell AP, Johnson-Ulrich Z, Lukas D, MacPherson M, Seitz BM, Sevchik A, Logan CJ | <p style="text-align: justify;">Research into animal cognitive abilities is increasing quickly and often uses methods where behavioral performance on a task is assumed to represent variation in the underlying cognitive trait. However, because thes... | Behaviour & Ethology, Evolutionary ecology, Preregistrations, Zoology | Aurélie Coulon | 2022-08-15 20:56:42 | View |
MANAGING BOARD
Julia Astegiano
Tim Coulson
Anna Eklof
Dominique Gravel
François Massol
Ben Phillips
Cyrille Violle










