
Reservoirs of pestilence: what pathogen and rodent community analyses can tell us about transmission risk
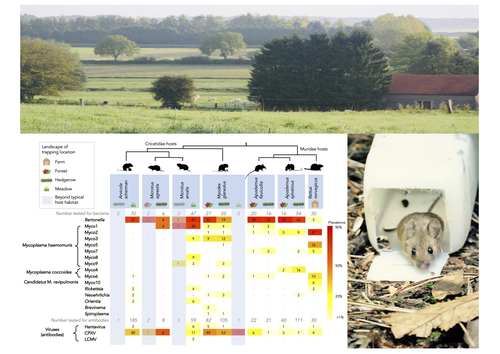
Pathogen community composition and co-infection patterns in a wild community of rodents
Abstract
Recommendation: posted 17 November 2023, validated 21 November 2023
Massol, F. (2023) Reservoirs of pestilence: what pathogen and rodent community analyses can tell us about transmission risk. Peer Community in Ecology, 100087. https://doi.org/10.24072/pci.ecology.100087
Recommendation
Rodents are well known as one of the main animal groups responsible for human-transmitted pathogens. As such, it seems logical to try and survey what kinds of pathogenic microbes might be harboured by wild rodents, in order to establish some baseline surveillance and prevent future zoonotic outbreaks (Bernstein et al., 2022). This is exactly what Abbate et al. (2023) endeavoured and their findings are intimidating. Based on quite a large sampling effort, they collected more than 700 rodents of seven species around two villages in northeastern France. They looked for molecular markers indicative of viral and bacterial infections and proceeded to analyze their pathogen communities using multivariate techniques.
Variation in the prevalence of the different pathogens was found among host species, with e.g. signs of CPXV more prevalent in Cricetidae while some Mycoplasma strains were more prevalent in Muridae. Co-circulation of pathogens was found in all species, with some evidencing signs of up to 12 different pathogen taxa. The diversity of co-circulating pathogens was markedly different between host species and higher in adult hosts, but not affected by sex. The dataset also evinced some slight differences between habitats, with meadows harbouring a little more diversity of rodent pathogens than forests. Less intuitively, some pathogen associations seemed quite repeatable, such as the positive association of Bartonella spp. with CPXV in the montane water vole. The study allowed the authors to test several associations already described in the literature, including associations between different hemotropic Mycoplasma species.
I strongly invite colleagues interested in zoonoses, emerging pandemics and more generally One Health to read the paper of Abbate et al. (2023) and try to replicate them across the world. To prevent the next sanitary crises, monitoring rodents, and more generally vertebrates, population demographics is a necessary and enlightening step (Johnson et al., 2020), but insufficient. Following the lead of colleagues working on rodent ectoparasites (Krasnov et al., 2014), we need more surveys like the one described by Abbate et al. (2023) to understand the importance of the dilution effect in the prevalence and transmission of microbial pathogens (Andreazzi et al., 2023) and the formation of epidemics. We also need other similar studies to assess the potential of different rodent species to carry pathogens more or less capable of infecting other mammalian species (Morand et al., 2015), in other places in the world.
References
Abbate, J. L., Galan, M., Razzauti, M., Sironen, T., Voutilainen, L., Henttonen, H., Gasqui, P., Cosson, J.-F. & Charbonnel, N. (2023) Pathogen community composition and co-infection patterns in a wild community of rodents. BioRxiv, ver.4 peer-reviewed and recommended by Peer Community in Ecology. https://doi.org/10.1101/2020.02.09.940494
Andreazzi, C. S., Martinez-Vaquero, L. A., Winck, G. R., Cardoso, T. S., Teixeira, B. R., Xavier, S. C. C., Gentile, R., Jansen, A. M. & D'Andrea, P. S. (2023) Vegetation cover and biodiversity reduce parasite infection in wild hosts across ecological levels and scales. Ecography, 2023, e06579.
https://doi.org/10.1111/ecog.06579
Bernstein, A. S., Ando, A. W., Loch-Temzelides, T., Vale, M. M., Li, B. V., Li, H., Busch, J., Chapman, C. A., Kinnaird, M., Nowak, K., Castro, M. C., Zambrana-Torrelio, C., Ahumada, J. A., Xiao, L., Roehrdanz, P., Kaufman, L., Hannah, L., Daszak, P., Pimm, S. L. & Dobson, A. P. (2022) The costs and benefits of primary prevention of zoonotic pandemics. Science Advances, 8, eabl4183.
https://doi.org/10.1126/sciadv.abl4183
Johnson, C. K., Hitchens, P. L., Pandit, P. S., Rushmore, J., Evans, T. S., Young, C. C. W. & Doyle, M. M. (2020) Global shifts in mammalian population trends reveal key predictors of virus spillover risk. Proceedings of the Royal Society B: Biological Sciences, 287, 20192736.
https://doi.org/10.1098/rspb.2019.2736
Krasnov, B. R., Pilosof, S., Stanko, M., Morand, S., Korallo-Vinarskaya, N. P., Vinarski, M. V. & Poulin, R. (2014) Co-occurrence and phylogenetic distance in communities of mammalian ectoparasites: limiting similarity versus environmental filtering. Oikos, 123, 63-70.
https://doi.org/10.1111/j.1600-0706.2013.00646.x
Morand, S., Bordes, F., Chen, H.-W., Claude, J., Cosson, J.-F., Galan, M., Czirjak, G. Á., Greenwood, A. D., Latinne, A., Michaux, J. & Ribas, A. (2015) Global parasite and Rattus rodent invasions: The consequences for rodent-borne diseases. Integrative Zoology, 10, 409-423.
https://doi.org/10.1111/1749-4877.12143
The recommender in charge of the evaluation of the article and the reviewers declared that they have no conflict of interest (as defined in the code of conduct of PCI) with the authors or with the content of the article. The authors declared that they comply with the PCI rule of having no financial conflicts of interest in relation to the content of the article.
x
Evaluation round #3
DOI or URL of the preprint: https://doi.org/10.1101/2020.02.09.940494
Version of the preprint: 3
Author's Reply, 15 Nov 2023
Please find our reply in the attached PDFs.
Sincerely,
Jessie Abbate
Decision by Francois Massol , posted 04 Sep 2023, validated 05 Sep 2023
, posted 04 Sep 2023, validated 05 Sep 2023
Dear authors,
thanks a lot for submitting your revised version of your ms. It is really good and I'll be delighted to recommend it soon. In the meantime, I pass you a commented/corrected version of the pdf so that you can make the paper "perfect" for the recommended version (you should have ~135 comments in the pdf; if not, please email me so that I can pass it on to you in another way).
On top of the comments in the pdf, here are some thoughts I jotted while reading:
1. A comment:
"However, it is unlikely any statistical approach can ever solve the problem of an unmeasured explanatory variable." (page 33, in the discussion)
This is the purpose of some latent variable or latent factor models, but it's true it is not always very well done. The literature on Structural Equation Models could be enlightening regarding this aspect. For a very general introduction to plausible causality inferred from observational data, I'd suggest reading the book of Pearl:
Pearl, J. (1988) Probabilistic Reasoning in Intelligent Systems: Networks of Plausible Inference, Morgan Kaufmann.
2. General suggestions on typos, formatting, etc.
* check some statistical terminology (e.g. "marginal means" in place of "contrasts"), I have pointed out some places in the text where it sounded weird, but you might also check for other terms (it never hurts);
* words in figures look misplaced sometimes -- check that the change from doc to pdf does not add some jittering of letter location;
* formatting of refences: remove double brackets, and if necessary insert square brackets inside normal ones (but not for references, use commas instead) -- this is easy to deal with this issue in some softwares (like endnote), but there's always the last resort possibility of transforming "code" (such as citation insertions) into pure text to format it as you wish;
* also regarding formatting references: no need to call for first names (or abbreviations of first names) when citing papers;
* (language) beware of the among/across difference. Among = between entities that are part of an identified group; across = throughout a whole range of (heterogeneous) entities. You could write "among host species" or "across the range of host species", but probably not "across host species". I've pointed some places where this is misused, but I certainly have missed issues like these.
Thanks again for all the work you have done.
Sincerely,
François Massol
Download recommender's annotationsEvaluation round #2
DOI or URL of the preprint: https://doi.org/10.1101/2020.02.09.940494
Version of the preprint: v1
Author's Reply, 22 Aug 2023
Attached you will find our responses, the tracked-changes version, and the PCI-formatted version.
Thank you and please let us know if you need anything else.
Decision by Francois Massol , posted 30 Mar 2021
, posted 30 Mar 2021
Dear authors,
thanks a lot for the thorough revisions you completed. As you will read, the two reviewers (one from last round of reviews, the other new) much appreciated the current version. I agree with them and I think that after some minor revisions (i.e. considering the points raised by R. Pigeault), your paper will be ready to be recommended.
I personally thank you for comparing the drop1 ANOVA tests with the model comparison procedure. However, from the results you show, I would really recommend using model comparisons (e.g. replacing drop1 tables by AICc tables of models or weight tables of variable importances) rather than drop1. From a purely statistical viewpoint, the comparisons that drop1 is making are not very relevant because they are grounded in the assumption that almost all tested variables should be included in the model. This can lead to spurious estimates of model coefficients, even if the "best model" and the drop1 tables seem to agree.
I hope I will soon read the revision of this excellent paper.
Sincerely,
François Massol
******
Additional comments from the Managing board
Mandatory modifications
1- Please make sure that:
-Data are available to readers, either in the text or through an open data repository such as Zenodo (free), Dryad or some other institutional repository. Data must be reusable, thus metadata or accompanying text must carefully describe the data. -Details on quantitative analyses (e.g., data treatment and statistical scripts in R, bioinformatic pipeline scripts, etc.) and details concerning simulations (scripts, codes) are available to readers in the text, as appendices, or through an open data repository, such as Zenodo, Dryad or some other institutional repository. The scripts or codes must be carefully described so that they can be reused. -Details on experimental procedures are available to readers in the text or as appendices. Include information about ethical approval for animal experimentation. Provide information about the compliance of their work with ethical standards of their national ethical committees and report the reference number of the ethical committee approval. If the study did not require ethical approval, include some sentences explaining why the approval was not needed.
-Authors have no financial conflict of interest relating to the article. The article must contain a "Conflict of interest disclosure" paragraph before the reference section containing this sentence: "The authors of this preprint declare that they have no financial conflict of interest with the content of this article." If appropriate, this disclosure may be completed by a sentence indicating that some of the authors are PCI recommenders: "XXX is one of the PCI Ecology recommenders."
2- Please make the following changes:
-Add the following sentence in the acknowledgements: "Version 3 of this preprint has been peer-reviewed and recommended by Peer Community In Ecology (https://doi.org/10.24072/pci.ecology.100071)"
-If you use bioRxiv to post your preprint, add this latter sentence also in the “revision summary” section of the deposit form of bioRxiv.
Note that this DOI is not the DOI of your article, but the DOI of the recommendation text. The DOI of your article remains unchanged.
3- If not yet done, please send us a picture for which you own the rights that could serve as a thumbnail/illustration for your article on the web site of PCI. It can be a figure of the article.
Optional instructions (we strongly advise you to follow them)
1- We suggest you to remove line numbering from the preprint and put the tables and figures within the text rather than at the end of your MS.
2- Then, we strongly advise you to use the PCI templates (word docx template or latex template) to format your preprint in a PCI style. Here is the links of the templates: https://peercommunityin.org/templates/
→ For word template:
Do not hesitate to modify the template as you want (and send it back to us if you made significant improvements).
-the text to be replaced by your own text starts with XXX, eg XXXXTitle of the article.
-XXXXthe "citeas" → Edeline, E. and Loeuille, N. (2021) Size-dependent eco-evolutionary feedbacks in fisheries. bioRxiv, 2020.04.03.022905, ver. 3 peer-reviewed and recommended by PCI Ecology. doi: https://doi.org/10.1101/2020.04.03.022905
-XXXXthe date of deposit in the preprint server → date of the deposit of the latest version
-XXXXthe surnames and names of the reviewers we sent you → Jean-François Arnoldi and an anonymous reviewer
-XXXXthedoiwesentyou → https://doi.org/10.24072/pci.ecology.100071
-XXXXthe surname and name of the recommender → Simon Blanchet
-In the acknowledgements, add this sentence → "Version 3 of this preprint has been peer-reviewed and recommended by Peer Community In Ecology (https://doi.org/10.24072/pci.ecology.100071)"
-Please be careful to choose the badges “Open Code” and “Open Data” only if appropriate (in addition to the “Open Access” and “Open Peer-Review” badges).
→ For Latex and mode org templates:
Do not hesitate to modify the template as you want (and send it back to us if you made significant improvements).
-main.tex and sample.bib should be filled.
-in main.tex, the recommender’s name is "Simon Blanchet" and the reviewers’ names are Jean-François Arnoldi and an anonymous reviewer -In sample.bib, indicate the right version of your preprint. It is version 3
-Preambuleecology.tex should be modified (comment lines 115, 119) to select badges. Please be careful to choose the badges “Open Code” and “Open Data” only if appropriate (in addition to the “Open Access” and “Open Peer-Review” badges).
3- we suggest that you deposit a copy of your MS in zenodo.org and ask for its inclusion in the PCI community (“Communities” section in the deposit form). Indicate the current doi of your MS, if it already has one, in the “doi” section.
Reviewed by Adrian Diaz, 29 Mar 2021
Dear Recommender,
Authors have address my comments and suggestions in a satisfactory way. So, I have no further comments for this manuscript.
https://doi.org/10.24072/pci.ecology.100087.rev21
Reviewed by Romain Pigeault, 17 Mar 2021
Dear Recommender,
In this manuscript authors aimed to characterize the parasite community harbouring by a wild community of rodents in France.
By combining different detection methods (molecular characterisation for pathogenic bacteria and antibody tests for viruses) and extensive statistical analyses, they explored how extrinsic (e.g. habitat, age, sex) and intrinsic factors shaped parasite community. They highlighted that host species is the most important factor of parasite community composition. Authors also confirm some specific pattern in parasite interactions (positive association) already published in literature.
The topic covered in this article is very interesting and relevant. I must say that I really enjoyed reading this manuscript. Although the article is very dense, it is very well written as well as very well structured, which makes it very enjoyable to read. The corrections/modifications made by the authors following the reviewers' comments seem to me to be quite appropriate. In reading the manuscript, I noted several limitations, related to the methodological and statistical approaches, but most of them are acknowledged and discussed by the authors. At this stage I have only a few minor comments and suggestions.
1) Although the authors state in the discussion that phylogeny has a minimal impact on pathogen diversity in rodent species, I would have been very curious to know the proportion of variance that is attributable to phylogeny and species identity respectively.
2) As the authors also seem to have individual data in addition to sex and age ("precise body measurements" lines 160-162), it would also have been interesting to study the impact of these parameters on parasite richness and individual parasite community composition (e.g. Mangombi et al 2021 10.1371/journal.pone.0248244).
3) Line 31: It would be useful to add (in brackets) the extrinsic factors.
4) Line 83: it would be relevant to cite the recent review published by Karvonen et al., 2019 (10.1016/j.pt.2018.11.007)
5) Lines 83-89: Mentioning the impact of co-infections on the evolution of virulence (Alizon et al 2013, 10.1111/ele.12076) and the maintenance of parasite genetic variation (and thus the impact on host/parasite coevolution, Seppälä et al 2016, 10.1016/j.pt.2016.08.010)might be relevant here?
6) Lines 101-102: I don't understand what "strong ecological interactions" refers to (competition and facilitation?).
7) Line 158: Is there a particular reason why the sampling was done in autumn? In rodents, seasonal changes can cause qualitative and quantitative differences in the gut microbiota (Maurice et al 2015, 10.1038/ismej.2015.53) but also in the composition of pathogenic bacterial communities (e.g. Kleynhans et al 2018, 10.1016/j.meegid.2018.07.036, Villette et al 2020, 10.1038/s41598-020-66107-5). Perhaps this limitation could be briefly mentioned (e.g. lines 718-721).
8) Line 160: Suggestion: use “age class” instead of “age”.
9) Line 169: How are the different habitats distributed in the two study sites?
10) Line 194: please add references.
11) Line220: please define chimera
12) Line 228: mention Table 1 here.
13) Table 1: This table is very useful. However, it would be “perfect” if there were also the references of the articles showing, or at least suggesting, the pathogenicity of the different bacteria. Also, in the 3rd column header, change "Nuimber" to "Number".
14) Line 235: I am not totally convinced by "co-exposure" (but I have no better suggestion...). In my opinion, "co-exposure" is associated with a time notion that cannot be captured by the approach used here (co-exposure = simultaneous exposure?).
15) Line 251: I agree with Recommender's comment about using drop1. However, the authors seem to want to keep this method.
16) Lines 370-371: It would be well to mention instead that R. norvegicus was not included in the first analysis (3.2.1)
17) Lines 452-458: I am not convinced that the two models here are really necessary. Given that R. norvegicus hosts had the second most diverse pathogen community and that only rats are present in farm habitats, it is quite obvious that farm habitats are among the habitats with the highest diversity.
18) Lines 466-475: More information is needed in the legend of figure S5. I can't make the connection between the result part and this figure.
19) Figure S2A: please add the horizontal bar mentioned in the caption or modify the caption.
20) Lines 530-642: I would like to congratulate the authors because despite the large number of tests performed (especially from part 3.3 onwards), the manuscript is really pleasant to read. The tables in the supInfo are really well structured and really help to understand. However, this requires a lot of back and forth between the MS and the SupInfo.
21) Line 691: “Here, we found to two distinct….” removed “to”
22) Line 800: add a full stop (.) at the end of the sentence.
23) Line 889: perhaps authors could add a reference here (e.g. Malmqvist et al 2004, 10.1098/rsbl.2003.0120 or Gutiérrez-López et al 2020, 10.3389/fevo.2020.569230)
24) Lines 984-987: please see e.g. Galen et al 2019, 10.1111/1365-2656.13089
25) It might be appropriate to point out in the limitations of this study that the detection of bacteria was only done in one organ (it is very likely that the authors missed other bacterial pathogens, please see: Mangombi et al 2021 10.1371/journal.pone.0248244, Genné et al 2021, 10.1038/s41396-021-00939-5).
https://doi.org/10.24072/pci.ecology.100087.rev22
Evaluation round #1
DOI or URL of the preprint: https://doi.org/10.1101/2020.02.09.940494
Author's Reply, 22 Feb 2021
Dear Recommender & Reviewers:
Please find our responses in red in the PDF attached, and line numbers refer to the tracked-changes document for your convenience.
We have updated the manuscript with substantial text edits based on reviewer comments and suggestions. However, there has been no qualitative change to any of the results, and conclusions remain unchanged, despite a few additional diversity analyses. Scripts and source data for analyses have been provided as supplemental materials and a few errors affecting quantitative values but not qualitative outcomes have been corrected. We believe the process was constructive and reassured us that our work was robust to the methods used.
The official clean version and all supplemental materials will be available on bioRxiv (screening completed) at https://www.biorxiv.org/content/10.1101/2020.02.09.940494v2
Sincerely, Jessie Abbate
Decision by Francois Massol , posted 26 Mar 2020
, posted 26 Mar 2020
Dear authors,
with two reviews and my own reading of your paper, I would like you to conduct a thorough revision before I can recommend your work. As you will see, both reviewers found a lot of merits to your work, but they also raised some concerns, notably regarding the limitations of the methods and their interpretation, as well as the lack of justification for some methodological choices.
Personally, I also find merits to your manuscript: the questions and data are sound, the general approach is interesting and the results are going to be quite useful for applied ecologists working on zoonotic prevention. However, you I have some issues with some of your methodological choices:
A simple one: PCI asks for data and code availability, but I have not been able to find any (or maybe my eyes don't work that well these days). Can you make R scripts and the raw data available for the next version of your manuscript?
For virus and bacterial detection, you use two different procedures (antibody detection and 16S amplification and sequencing). However, these are not only technical differences, but also epistemic ones: with antibodies, you are only going to find viruses that you were looking for, while with 16S you can find bacteria that you even had not suspected in the first place. For this reason, many of the analyses will not behave in the same way for viruses and bacteria. I suggest that you acknowledge this early on (in the M&M) and discuss it thoroughly.
While I understand your focus on pathogenic bacteria and viruses, it would probably be interesting to also look at other forms of symbiont association (negative or positive), for instance non-pathogenic bacteria with pathogenic ones, non-pathogenic viruses with pathogenic bacteria, etc. You obviously did not search for non-pathogenic viruses like bacteriophages, but at least if you still have blasted data on non-pathogenic bacteria, there might be something to dig in there about microbial associations within rodents (notably, it would be cool to know whether some other bacteria are negatively associated with nasty pathogens).
Quite a large part of the Introduction announces apicomplexan, protozoa, cestods, trematods, etc. as important parasites of rodents, but you chose to focus on bacteria and viruses. Why? (I guess for money reason, but maybe you can elaborate)
For the diversity analyses, did you use multiplicative or additive Shannon numbers? (i.e. the exponential version or the log version) As you considered both beta and alpha diversities, which version of the partitioning did you use? (as there are at least two main ones, Jost-Routledge's and Chao's) Did you correct for the usual bias in estimates of Shannon diversity? (using e.g. Chao's estimator) I think the entropart package does this...
I'm not a big fan of the drop1 method (i.e. likelihood ratio test with the complete model only) because the best model explaining your data can actually have fewer than n-1 variables in it (if n is the total of all tested variables). A very simple trick would be to run your complete model through GLMulti to get the AIC or BIC of all models and ascertain which one is the best. That way, you could have a plausible answer to the question "which factors affect Shannon diversity of pathogens?".
At some point you mention that you wanted to relate beta diversities with factors, but I did not see that exactly. An option to do this easily is to use dissimilarities obtained from beta diversities (see e.g. Ohlmann et al. 2019) as a distance and then make an adonis analysis on these distances using factors as covariables (adonis is in package vegan). That way, you could explain differences in pathogen composition using some of your factors (habitats...).
Fig. 2 has an issue: I don't think you can test anything using residuals. If you delete one factor in a model and then plot the residuals according to the modalities of that dismissed factor, what you will see is only how bad your model is at compensating this missing factor, not the intrinsic differences between modalities. To obtain the differences between modalities, you need to actually predict the model (with the factor you want to look at) on new data (using predict in R), probably with a randomization (bootstrap) of the initial data.
In Fig. 3B and around it: when you write "pathogen co-exposure", does it mean "virus + bacteria co-infections"?
Regarding the "MCA -> principal dimensions to be regressed individually by factors" approach, I'm sure you can do better than that by using CCA (Peres-Neto et al. 2006) or distance-based RDA (Blanchet et al. 2014) to obtain the fractions of the total chi² of your host x pathogen table explained by each factor. The individual regression of MCA eigenvector seems a bit pedestrian and it does not give a super-clear answer in the end.
Fig. 4 does not seem that useful -- do you really need it?
As stated above about model comparisons through AIC or BIC using GLMulti, I suggest you do something like that for the GLM you do on pathos-pathos associations.
Or maybe better: for this part, you can also shift completely and use your dataset as a network (host nodes connected to pathos nodes). By using any sensible community-detection algorithm or block model, you can find modules/blocks/communities of pathogens that have similar connection patterns, which in a way is what you are looking for, isn't it? This approach is less driven by hypotheses (it's actually super-exploratory), but it might confirm what you know while at the same time finding associations you did not see or did not expect. I suggest Leger et al. (2015) as a good reading on modules/blocks in networks, if needed.
Or you can do both: GLMs to test particular associations and module-search to get a broader view on all associations...
A very general comment: you mention p-value corrections only for Tukey's tests, but given the amount of tests performed, I suggest you use p.adjust with Benjamini-Hochberg correction overall (so you do that for the vector of all p-values obtained with all analyses on the same dataset).
The discussion is a bit long (9 pages +), I guess you can shorten the parts where you repeat the results.
Typos/minor things L. 90 Leptospirosa => Leptospira ? L. 133 sexually-immature => sexually immature L. 208 "This produced a down to a set of quantitative (...)" The sentence looks warped. L. 252 implicated => involved ? L. 276-277 For M&M instead? L. 290-297 Problems with punctuation and coherence between propositions: "because they were...", "inability to rule out...", "or because their identity..." L. 335 pseudo-R² from deviance ? Please state it. L. 424 "more rarely than expected (p = 0.13..." -- really expected? A p-value above 0.05 more likely means "as expected"
I hope you will find all reviews useful for revising your paper. Sincerely,
François Massol
Cited references Blanchet, F. G., Legendre, P., Bergeron, J. A. C. & He, F. (2014) Consensus RDA across dissimilarity coefficients for canonical ordination of community composition data. Ecological Monographs, 84, 491-511.
Chao, A. & Chiu, C.-H. (2016) Bridging the variance and diversity decomposition approaches to beta diversity via similarity and differentiation measures. Methods in Ecology and Evolution, 7, 919-928.
Chao, A., Wang, Y. T. & Jost, L. (2013) Entropy and the species accumulation curve: a novel entropy estimator via discovery rates of new species. Methods in Ecology and Evolution, 4, 1091-1100.
Jost, L. (2007) Partitioning diversity into independent alpha and beta components. Ecology, 88, 2427-2439.
Leger, J.-B., Daudin, J.-J. & Vacher, C. (2015) Clustering methods differ in their ability to detect patterns in ecological networks. Methods in Ecology and Evolution, 6, 474-481.
Ohlmann, M., Miele, V., Dray, S., Chalmandrier, L., O’Connor, L. & Thuiller, W. (2019) Diversity indices for ecological networks: a unifying framework using Hill numbers. Ecology Letters, 22, 737-747.
Peres-Neto, P. R., Legendre, P., Dray, S. & Borcard, D. (2006) Variation partitioning of species data matrices: Estimation and comparison of fractions. Ecology, 87, 2614-2625.
Routledge, R. D. (1979) Diversity indices: Which ones are admissible? Journal of Theoretical Biology, 76, 503-515.
Reviewed by anonymous reviewer 1, 05 Mar 2020
The authors present a study entitled "Pathogen community composition and co-infection patterns in a wild community of rodents" in which they analyze rodent-borne pathogen communities in a rural area of northern France, a region known to be endemic for several rodent-borne diseases. Using elegant, stepwise and pedagogically described approaches, the authors propose that the host species is the main determinant of pathogen community composition and that hosts share habitats that may have very different pathogen communities. The authors are aware of the weak points of their study and hypotheses are always made in relation to them. The figures are very clear and very esthetic. All the results obtained lead to a very descriptive study with a good number of speculations that need to be confirmed by experimental studies.
If I go back to the order of the article:
Line 104-107 "We investigate exposure ......metbarcoding) : Why distinguish between the two? How can we be sure that there is no old bacterial or recent viral association?
Line 160 : Why choose the spleen as the only tissue to be analyzed?
Line 203 : "to regroup significantly distinct factor levels" Isn't it the other way around, i.e. grouping levels that are not significantly different?
Line 223 : "where evidence suggested" I don't understand what evidence are the authors talking about?
Line 255-256 : (-2log.....full model) explanation to move line 199 when the function "drop1" is mentioned for the first time?
Line 256-258: "Despite...sampled" Why this choice when the risk of false positive is high?
Line 270-271 : "No animals...antibodies" This sentence seems incomplete to me
Line 458 : "antibodies: x2=5.21"to be completed by "antibodies - Bartonella: x2=5.21"
Line 471: "non-significant interaction...."In the material and method, it is not indicated that interactions are included.
Reviewed by Adrian Diaz, 23 Mar 2020
Dear Recommender, The manuscript entitled "Pathogen community composition and co-infection patterns in a wild community of rodents" submitted by Jessica L. Abbate et al to PCI Ecology aimed to characterize the pathogen community harboring by a wild community of rodents in north of France. Through seroprevalence techniques and molecular characterization, authors studied the community composition, pattern of distribution of pathogens in the host species range and habitats. Also, through appropriate statistical analysis, they evaluate how intrinsic and extrinsic factors influence the pathogen activity/distribution. Moreover, they tested a priori hypothesis regarding pathogen interactions already published in scientific literature and a plus of potential interactions detected during the study. Although the pathogen detection/identification methodology is quite assertive they had limitations and those are clearly stated at the end of Discussion section:
General comments:
Overall the manuscript was well written, including a detailed statistical analyses section and a good graphical support of results sometimes, specially in the Discussion section the English is hard to follow. I recommend the authors to revise in order to make it more fluent for readers.
Abstract, Line 25: Authors expressed they study the incidence of pathogens in the rodents community. Actually they estimate the prevalence or presence/absence of pathogen in the community. Incidence means how many individuals changed their status as a function of time (diseases, infection,)
If you have seroprevalence data with no assumption about time of exposure. Can you associate that with an acute infection? That could result in a spurious speculation with no biological meaning?
How did you build your matrix in the pathogen community composition?
Page 19 Lines 427-430. When you ran GLM tests to analyze Myco1, Myco3 and anti-hantaviruses antibodies interactions you stated extrinsic factors as drivers and also as heterogenous host group. It is not clear.
Did you consider microbiome (OTUs from microbes typically of healthy flora) as an intrinsic factor driving pathogen diversity?
Minor editing observations:
Page 11 Line 264. Better state the Number of positive sera and the percentage
Figure 3 B and 3 C: Is Pathogen equal to virus or mixing viruses and bacteria? In the first case states virus rather than pathogen since you have been using that criteria.
Figure 5. Better state anti xxxx antibodies to homogenize it use through the manuscript.
Discusssion: Page 23, Line 501: Where were Lyjungan virus and spirochetes detected? In your study? Please clarify.
Page 24, Line 532: Could you please clarify this sentence “has only ever before been”
Poor discussion about why some host species harbor poor number of zoonotic agents.
First two pages are describing patterns already described in results. It sounds a little redundant.
Page 26, Line 578: change colom by a period.
Do you test any association about diversity of co-infection and diversity of host species at place?
Page 28, Line 650: anti-hantaviurs antibody
Page 28, line 668: (Previously described by…)
Page 29, Line 654: association between Mycoplasma and anti-hantaviruses antibodies. Authors stated this positive interaction is because of the cronic diseases that infectious agents developed. In that
Page 29, Lines 656-657: why independently of host species identity?
Page 29, Line 662: anti-CPXV antibodies
Page 29, Line 664: delete virus. It is already included in the abbreviation CPXV
Page 29, Line 678: reported instead of revealed
Page 32, Line 740: undescribed
https://doi.org/10.24072/pci.ecology.100087.rev12









