DIAZ Adrian
- Laboratorio de Arbovirus, Instituto de Virología "Dr. J. M. Vanella" - Universidad Nacional de Córdoba / Instituto de Investigaciones Biológicas y Tecnológicas - CONICET, Córdoba, Argentina
- Epidemiology, Host-parasite interactions, Microbial ecology & microbiology, Parasitology
- recommender
Recommendation: 1
Review: 1
Recommendation: 1
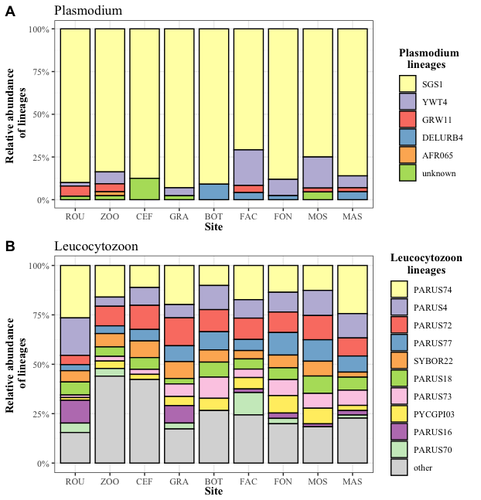
Cities as parasitic amplifiers? Malaria prevalence and diversity in great tits along an urbanization gradient
Exploring the Impact of Urbanization on Avian Malaria Dynamics in Great Tits: Insights from a Study Across Urban and Non-Urban Environments
Recommended by Adrian Diaz based on reviews by Ana Paula Mansilla and 2 anonymous reviewersAcross the temporal expanse of history, the impact of human activities on global landscapes has manifested as a complex interplay of ecological alterations. From the advent of early agricultural practices to the successive waves of industrialization characterizing the 18th and 19th centuries, anthropogenic forces have exerted profound and enduring transformations upon Earth's ecosystems. Indeed, by 2017, more than 80% of the terrestrial biosphere was transformed by human populations and land use, and just 19% remains as wildlands (Ellis et al. 2021).
Urbanization engenders profound alterations in environmental conditions, exerting substantial impacts on biological communities. The expansion of built infrastructure, modification of land use patterns, and the introduction of impervious surfaces and habitat fragmentation are key facets of urbanization (Faeth et al. 2011). These alterations generate biodiversity loss, changes in the composition of biological communities, disruptions in access and availability of food and nutrients, and a loss of efficiency in the immune system's control of infections, etc. (Reyes et al. 2013).
In this study, Caizergues et al. (2023) investigated the prevalence and diversity of avian malaria parasites (Plasmodium/Haemoproteus sp. and Leucocytozoon sp.) in great tits (Parus major) living across an urbanization gradient. The study reveals nuanced patterns of avian malaria prevalence and lineage diversity in great tits across urban and non-urban environments. While overall parasite diversity remains consistent, there are marked differences in prevalence between life stages and habitats. They observed a high prevalence in adult birds (from 95% to 100%), yet lower prevalence in fledglings (from 0% to 38%). Notably, urban nestlings exhibit higher parasite prevalence than their non-urban counterparts, suggesting a potential link between early malaria infection and the urban heat island effect. This finding underscores the importance of considering both spatial and temporal aspects of urbanization in understanding disease dynamics. Parasite lineages were not habitat-specific. The results suggest a potential parasitic burden in more urbanized areas, with a marginal but notable effect of nest-level urbanization on Plasmodium prevalence. This challenges the common perception of lower parasitic prevalence in urban environments and highlights the need for further investigation into the factors influencing parasite prevalence at finer spatial scales.
The discussion emphasizes the significance of examining vector distributions, abundance, and diversity in urban areas, which may be influenced by ecological niches and the presence of suitable habitats such as marshes. The identification of habitat-specific Haemosporidian lineages, particularly those occurring more frequently in urban areas, raises intriguing questions about the factors influencing parasite diversity. The presence of rare lineages in urban environments, such as AFR065, DELURB4, and YWT4, suggests a potential connection between urban bird communities and specific parasite strains.
Future research should empirically demonstrate these relationships to enhance our understanding of urban parasitology. This finding has broader implications for wildlife epidemiology, especially when introducing or keeping exotic wildlife in contact with native species. The study highlights the importance of considering not only the prevalence but also the specific lineages of parasites in understanding the dynamics of avian malaria in urban and non-urban habitats. This preprint contributes valuable insights to the ongoing discourse on the intricate interplay between ecological repercussions of human-induced changes (urbanization), biological communities, and the prevalence of vector-borne diseases.
References
Caizergues AE, Robira B, Perrier C, Jeanneau M, Berthomieu A, Perret S, Gandon S, Charmantier A (2023) Cities as parasitic amplifiers? Malaria prevalence and diversity in great tits along an urbanization gradient. bioRxiv, 2023.05.03.539263, ver. 3 peer-reviewed and recommended by Peer Community in Ecology. https://doi.org/10.1101/2023.05.03.539263
Ellis EC, Gauthier N, Klein Goldewijk K, Bliege Bird R, Boivin N, Díaz S, Fuller DQ, Gill JL, Kaplan JO, Kingston N, Locke H, McMichael CNH, Ranco D, Rick TC, Shaw MR, Stephens L, Svenning JC, Watson JEM. People have shaped most of terrestrial nature for at least 12,000 years. Proc Natl Acad Sci U S A. 2021 Apr 27;118(17):e2023483118. https://doi.org/10.1073/pnas.2023483118.
Faeth SH, Bang C, Saari S (2011) Urban biodiversity: Patterns and mechanisms. Ann N Y Acad Sci 1223:69–81. https://doi.org/10.1111/j.1749-6632.2010.05925.x
Faeth SH, Bang C, Saari S (2011) Urban biodiversity: Patterns and mechanisms. Ann N Y Acad Sci 1223:69–81. https://doi.org/10.1111/j.1749-6632.2010.05925.x
Reyes R, Ahn R, Thurber K, Burke TF (2013) Urbanization and Infectious Diseases: General Principles, Historical Perspectives, and Contemporary Challenges. Challenges Infect Dis 123. https://doi.org/10.1007/978-1-4614-4496-1_4
Review: 1
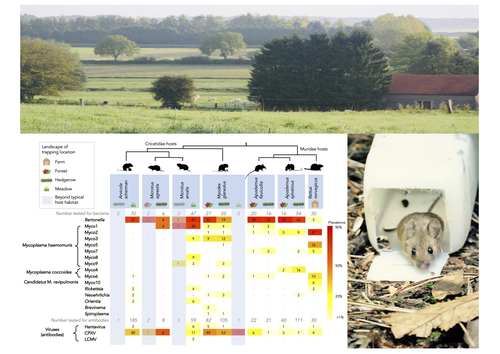
Pathogen community composition and co-infection patterns in a wild community of rodents
Reservoirs of pestilence: what pathogen and rodent community analyses can tell us about transmission risk
Recommended by Francois Massol based on reviews by Adrian Diaz, Romain Pigeault and 1 anonymous reviewerRodents are well known as one of the main animal groups responsible for human-transmitted pathogens. As such, it seems logical to try and survey what kinds of pathogenic microbes might be harboured by wild rodents, in order to establish some baseline surveillance and prevent future zoonotic outbreaks (Bernstein et al., 2022). This is exactly what Abbate et al. (2023) endeavoured and their findings are intimidating. Based on quite a large sampling effort, they collected more than 700 rodents of seven species around two villages in northeastern France. They looked for molecular markers indicative of viral and bacterial infections and proceeded to analyze their pathogen communities using multivariate techniques.
Variation in the prevalence of the different pathogens was found among host species, with e.g. signs of CPXV more prevalent in Cricetidae while some Mycoplasma strains were more prevalent in Muridae. Co-circulation of pathogens was found in all species, with some evidencing signs of up to 12 different pathogen taxa. The diversity of co-circulating pathogens was markedly different between host species and higher in adult hosts, but not affected by sex. The dataset also evinced some slight differences between habitats, with meadows harbouring a little more diversity of rodent pathogens than forests. Less intuitively, some pathogen associations seemed quite repeatable, such as the positive association of Bartonella spp. with CPXV in the montane water vole. The study allowed the authors to test several associations already described in the literature, including associations between different hemotropic Mycoplasma species.
I strongly invite colleagues interested in zoonoses, emerging pandemics and more generally One Health to read the paper of Abbate et al. (2023) and try to replicate them across the world. To prevent the next sanitary crises, monitoring rodents, and more generally vertebrates, population demographics is a necessary and enlightening step (Johnson et al., 2020), but insufficient. Following the lead of colleagues working on rodent ectoparasites (Krasnov et al., 2014), we need more surveys like the one described by Abbate et al. (2023) to understand the importance of the dilution effect in the prevalence and transmission of microbial pathogens (Andreazzi et al., 2023) and the formation of epidemics. We also need other similar studies to assess the potential of different rodent species to carry pathogens more or less capable of infecting other mammalian species (Morand et al., 2015), in other places in the world.
References
Abbate, J. L., Galan, M., Razzauti, M., Sironen, T., Voutilainen, L., Henttonen, H., Gasqui, P., Cosson, J.-F. & Charbonnel, N. (2023) Pathogen community composition and co-infection patterns in a wild community of rodents. BioRxiv, ver.4 peer-reviewed and recommended by Peer Community in Ecology. https://doi.org/10.1101/2020.02.09.940494
Andreazzi, C. S., Martinez-Vaquero, L. A., Winck, G. R., Cardoso, T. S., Teixeira, B. R., Xavier, S. C. C., Gentile, R., Jansen, A. M. & D'Andrea, P. S. (2023) Vegetation cover and biodiversity reduce parasite infection in wild hosts across ecological levels and scales. Ecography, 2023, e06579.
https://doi.org/10.1111/ecog.06579
Bernstein, A. S., Ando, A. W., Loch-Temzelides, T., Vale, M. M., Li, B. V., Li, H., Busch, J., Chapman, C. A., Kinnaird, M., Nowak, K., Castro, M. C., Zambrana-Torrelio, C., Ahumada, J. A., Xiao, L., Roehrdanz, P., Kaufman, L., Hannah, L., Daszak, P., Pimm, S. L. & Dobson, A. P. (2022) The costs and benefits of primary prevention of zoonotic pandemics. Science Advances, 8, eabl4183.
https://doi.org/10.1126/sciadv.abl4183
Johnson, C. K., Hitchens, P. L., Pandit, P. S., Rushmore, J., Evans, T. S., Young, C. C. W. & Doyle, M. M. (2020) Global shifts in mammalian population trends reveal key predictors of virus spillover risk. Proceedings of the Royal Society B: Biological Sciences, 287, 20192736.
https://doi.org/10.1098/rspb.2019.2736
Krasnov, B. R., Pilosof, S., Stanko, M., Morand, S., Korallo-Vinarskaya, N. P., Vinarski, M. V. & Poulin, R. (2014) Co-occurrence and phylogenetic distance in communities of mammalian ectoparasites: limiting similarity versus environmental filtering. Oikos, 123, 63-70.
https://doi.org/10.1111/j.1600-0706.2013.00646.x
Morand, S., Bordes, F., Chen, H.-W., Claude, J., Cosson, J.-F., Galan, M., Czirjak, G. Á., Greenwood, A. D., Latinne, A., Michaux, J. & Ribas, A. (2015) Global parasite and Rattus rodent invasions: The consequences for rodent-borne diseases. Integrative Zoology, 10, 409-423.
https://doi.org/10.1111/1749-4877.12143