Latest recommendations

| Id | Title | Authors | Abstract▼ | Picture | Thematic fields | Recommender | Reviewers | Submission date | |
|---|---|---|---|---|---|---|---|---|---|
10 Jan 2024
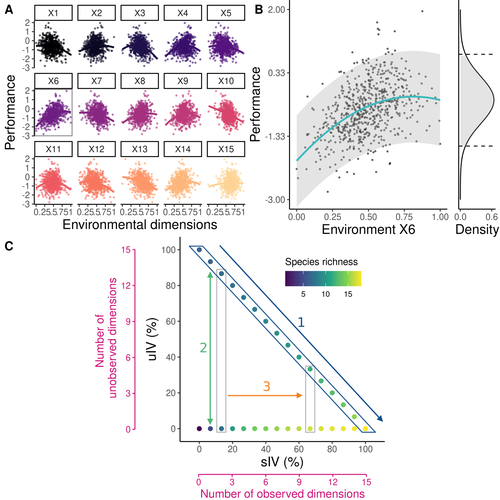
Beyond variance: simple random distributions are not a good proxy for intraspecific variability in systems with environmental structureCamille Girard-Tercieux, Ghislain Vieilledent, Adam Clark, James S. Clark, Benoit Courbaud, Claire Fortunel, Georges Kunstler, Raphaël Pélissier, Nadja Rüger, Isabelle Maréchaux https://doi.org/10.1101/2022.08.06.503032Two paradigms for intraspecific variabilityRecommended by Matthieu Barbier based on reviews by Simon Blanchet and Bart Haegeman based on reviews by Simon Blanchet and Bart Haegeman
Community ecology usually concerns itself with understanding the causes and consequences of diversity at a given taxonomic resolution, most classically at the species level. Yet there is no doubt that diversity exists at all scales, and phenotypic variability within a taxon can be comparable to differences between taxa, as observed from bacteria to fish and trees. The question that motivates an active and growing body of work (e.g. Raffard et al 2019) is not so much whether intraspecific variability matters, but what we get wrong by ignoring it and how to incorporate it into our understanding of communities. There is no established way to think about diversity at multiple nested taxonomic levels, and it is tempting to summarize intraspecific variability simply by measuring species mean and variance in any trait and metric. In this study, Girard-Tercieux et al (2023a) propose that, to understand its impact on community-level outcomes and in particular on species coexistence, we should carefully distinguish between two ways of thinking about intraspecific variability: -"unstructured" variation, where every individual's features are like an independent random draw from a species-specific distribution, for instance, due to genetic lottery and developmental accidents -"structured" variation that is due to each individual encountering a different but enduring microenvironment. The latter type of variability may still appear complex and random-like when the environment is high-dimensional (i.e. multifaceted, with many different factors contributing to each individual's performance and development). Thus, it is not necessarily "structured" in the sense of being easily understood -- we may need to measure more aspects of the environment than is practical if we want to fully predict these variations. What distinguishes this "structured" variability is that it is, in a loose sense, inheritable: individuals from the same species that grow in the same microenvironment will have the same performance, in a repeatable fashion. Thus, if each species is best at exploiting at least a fraction of environmental conditions, it is likely to avoid extinction by competition, except in the unlucky case of no propagule reaching any of the favorable sites. The core intuition, that the complex spatial structure and high-dimensional nature of the environment plays a key explanatory role in species coexistence, is a running thread through several of the authors' work (e.g. Clark et al 2010), clearly inspired by their focus on tropical forests. This study, by tackling the question of intraspecific determinants of interspecific outcomes, makes a compelling addition to this line of investigation, coming as a theoretical companion to a more data-oriented study (Girard-Tercieux et al 2023b). But I believe it raises a question that is even broader in scope. This kind of intraspecific variability, due to different individuals growing in different microenvironments, is perhaps most relevant for trees and other sessile organisms, but the distinction made here between "unstructured" and "structured" variability can likely be extended to many other ecological settings. In my understanding, what matters most in "structured" variability is not so much it stemming from a fixed environment, but rather it being maintained across generations, rather than possibly lost by drift. This difference between variability in the form of "frozen" randomness and in the form of stochastic drift over time is highly relevant in other theoretical fields (e.g. in physics, where it is the difference between a disordered solid and a liquid), and thus, I expect that it is a meaningful distinction to make throughout community ecology. References James S. Clark, David Bell, Chengjin Chu, Benoit Courbaud, Michael Dietze, Michelle Hersh, Janneke HilleRisLambers et al. (2010) "High‐dimensional coexistence based on individual variation: a synthesis of evidence." Ecological Monographs 80, no. 4 : 569-608. https://doi.org/10.1890/09-1541.1 Camille Girard-Tercieux, Ghislain Vieilledent, Adam Clark, James S. Clark, Benoît Courbaud, Claire Fortunel, Georges Kunstler, Raphaël Pélissier, Nadja Rüger, Isabelle Maréchaux (2023a) "Beyond variance: simple random distributions are not a good proxy for intraspecific variability in systems with environmental structure." bioRxiv, ver. 4 peer-reviewed and recommended by Peer Community in Ecology. https://doi.org/10.1101/2022.08.06.503032 Camille Girard‐Tercieux, Isabelle Maréchaux, Adam T. Clark, James S. Clark, Benoît Courbaud, Claire Fortunel, Joannès Guillemot et al. (2023b) "Rethinking the nature of intraspecific variability and its consequences on species coexistence." Ecology and Evolution 13, no. 3 : e9860. https://doi.org/10.1002/ece3.9860 Allan Raffard, Frédéric Santoul, Julien Cucherousset, and Simon Blanchet. (2019) "The community and ecosystem consequences of intraspecific diversity: A meta‐analysis." Biological Reviews 94, no. 2: 648-661. https://doi.org/10.1111/brv.12472 | Beyond variance: simple random distributions are not a good proxy for intraspecific variability in systems with environmental structure | Camille Girard-Tercieux, Ghislain Vieilledent, Adam Clark, James S. Clark, Benoit Courbaud, Claire Fortunel, Georges Kunstler, Raphaël Pélissier, Nadja Rüger, Isabelle Maréchaux | <p>The role of intraspecific variability (IV) in shaping community dynamics and species coexistence has been intensively discussed over the past decade and modelling studies have played an important role in that respect. However, these studies oft... |  | Biodiversity, Coexistence, Community ecology, Competition, Theoretical ecology | Matthieu Barbier | 2022-08-07 12:51:30 | View | |
07 Aug 2023
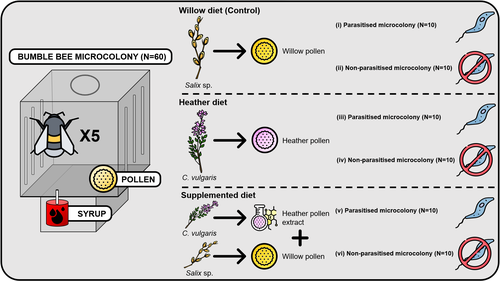
Heather pollen is not necessarily a healthy diet for bumble beesClément Tourbez, Irène Semay, Apolline Michel, Denis Michez, Pascal Gerbaux, Antoine Gekière, Maryse Vanderplanck https://doi.org/10.5281/zenodo.8192036The importance of understanding bee nutritionRecommended by Ignasi Bartomeus based on reviews by Cristina Botías and 1 anonymous reviewer based on reviews by Cristina Botías and 1 anonymous reviewer
Contrasting with the great alarm on bee declines, it is astonishing how little basic biology we know about bees, including on abundant and widespread species that are becoming model species. Plant-pollinator relationships are one of the cornerstones of bee ecology, and researchers are increasingly documenting bees' diets. However, we rarely know which effects feeding on different flowers has on bees' health. This paper (Tourbez et al. 2023) uses an elegant experimental setting to test the effect of heather pollen on bumblebees' (Bombus terrestris) reproductive success. This is a timely question as heather is frequently used by bumblebees, and its nectar has been reported to reduce parasite infections. In fact, it has been suggested that bumblebees can medicate themselves when infected (Richardson et al. 2014), and the pollen of some Asteraceae has been shown to help them fight parasites (Gekière et al. 2022). The starting hypothesis is that heather pollen contains flavonoids that might have a similar effect. Unfortunately, Tourbez and collaborators do not support this hypothesis, showing a negative effect of heather pollen, in particular its flavonoids, in bumblebees offspring, and an increase in parasite loads when fed on flavonoids. This is important because it challenges the idea that many pollen and nectar chemical compounds might have a medicinal use, and force us to critically analyze the effect of chemical compounds in each particular case. The results open several questions, such as why bumblebees collect heather pollen, or in which concentrations or pollen mixes it is deleterious. A limitation of the study is that it uses micro-colonies, and extrapolating this to real-world conditions is always complex. Understanding bee declines require a holistic approach starting with bee physiology and scaling up to multispecies population dynamics. References Gekière, A., Semay, I., Gérard, M., Michez, D., Gerbaux, P., & Vanderplanck, M. 2022. Poison or Potion: Effects of Sunflower Phenolamides on Bumble Bees and Their Gut Parasite. Biology, 11(4), 545. https://doi.org/10.3390/biology11040545 Richardson, L.L., Adler, L.S., Leonard, A.S., Andicoechea, J., Regan, K.H., Anthony, W.E., Manson, J.S., & Irwin, R.E. 2015. Secondary metabolites in floral nectar reduce parasite infections in bumblebees. Proceedings of the Royal Society of London B: Biological Sciences 282 (1803), 20142471. https://doi.org/10.1098/rspb.2014.2471 Tourbez, C., Semay, I., Michel, A., Michez, D., Gerbaux, P., Gekière A. & Vanderplanck, M. 2023. Heather pollen is not necessarily a healthy diet for bumble bees. Zenodo, ver 3, reviewed and recommended by PCI Ecology. https://doi.org/10.5281/zenodo.8192036 | Heather pollen is not necessarily a healthy diet for bumble bees | Clément Tourbez, Irène Semay, Apolline Michel, Denis Michez, Pascal Gerbaux, Antoine Gekière, Maryse Vanderplanck | <p>There is evidence that specialised metabolites of flowering plants occur in both vegetative parts and floral resources (i.e., pollen and nectar), exposing pollinators to their biological activities. While such metabolites may be toxic to bees, ... |  | Botany, Chemical ecology, Host-parasite interactions, Pollination, Zoology | Ignasi Bartomeus | 2023-04-10 21:22:34 | View | |
17 May 2023
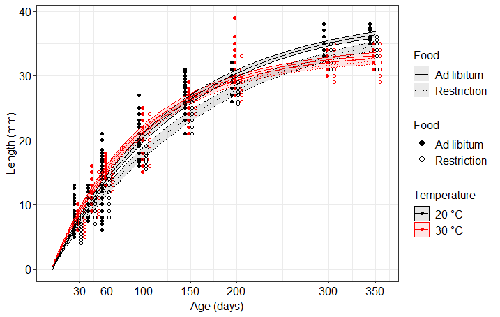
Distinct impacts of food restriction and warming on life history traits affect population fitness in vertebrate ectothermsSimon Bazin, Claire Hemmer-Brepson, Maxime Logez, Arnaud Sentis, Martin Daufresne https://hal.inrae.fr/hal-03738584v3Effect of food conditions on the Temperature-Size RuleRecommended by Aleksandra Walczyńska based on reviews by Wolf Blanckenhorn and Wilco VerberkTemperature-size rule (TSR) is a phenomenon of plastic changes in body size in response to temperature, originally observed in more than 80% of ectothermic organisms representing various groups (Atkinson 1994). In particular, ectotherms were observed to grow faster and reach smaller size at higher temperature and grow slower and achieve larger size at lower temperature. This response has fired the imagination of researchers since its invention, due to its counterintuitive pattern from an evolutionary perspective (Berrigan and Charnov 1994). The main question to be resolved is: why do organisms grow fast and achieve smaller sizes under more favourable conditions (= relatively higher temperature), while they grow longer and achieve larger sizes under less favourable conditions (relatively lower temperature), if larger size means higher fitness, while longer development may be risky? This evolutionary conundrum still awaits an ultimate explanation (Angilletta Jr et al. 2004; Angilletta and Dunham 2003; Verberk et al. 2021). Although theoretical modelling has shown that such a growth pattern can be achieved as a response to temperature alone, with a specific combination of energetic parameters and external mortality (Kozłowski et al. 2004), it has been suggested that other temperature-dependent environmental variables may be the actual drivers of this pattern. One of the most frequently invoked variable is the relative oxygen availability in the environment (e.g., Atkinson et al. 2006; Audzijonyte et al. 2019; Verberk et al. 2021; Woods 1999), which decreases with temperature increase. Importantly, this effect is more pronounced in aquatic systems (Forster et al. 2012). However, other temperature-dependent parameters are also being examined in the context of their possible effect on TSR induction and strength. Food availability is among the interfering factors in this regard. In aquatic systems, nutritional conditions are generally better at higher temperature, while a range of relatively mild thermal conditions is considered. However, there are no conclusive results so far on how nutritional conditions affect the plastic body size response to acute temperature changes. A study by Bazin et al. (2023) examined this particular issue, the effects of food and temperature on TSR, in medaka fish. An important value of the study was to relate the patterns found to fitness. This is a rare and highly desirable approach since evolutionary significance of any results cannot be reliably interpreted unless shown as expressed in light of fitness. The authors compared the body size of fish kept at 20°C and 30°C under conditions of food abundance or food restriction. The results showed that the TSR (smaller body size at 30°C compared to 20°C) was observed in both food treatments, but the effect was delayed during fish development under food restriction. Regarding the relevance to fitness, increased temperature resulted in more eggs laid but higher mortality, while food restriction increased survival but decreased the number of eggs laid in both thermal treatments. Overall, food restriction seemed to have a more severe effect on development at 20°C than at 30°C, contrary to the authors’ expectations. I found this result particularly interesting. One possible interpretation, also suggested by the authors, is that the relative oxygen availability, which was not controlled for in this study, could have affected this pattern. According to theoretical predictions confirmed in quite many empirical studies so far, oxygen restriction is more severe at higher temperatures. Perhaps for these particular two thermal treatments and in the case of the particular species studied, this restriction was more severe for organismal performance than the food restriction. This result is an example that all three variables, temperature, food and oxygen, should be taken into account in future studies if the interrelationship between them is to be understood in the context of TSR. It also shows that the reasons for growing smaller in warm may be different from those for growing larger in cold, as suggested, directly or indirectly, in some previous studies (Hessen et al. 2010; Leiva et al. 2019). Since medaka fish represent predatory vertebrates, the results of the study contribute to the issue of global warming effect on food webs, as the authors rightly point out. This is an important issue because the general decrease in the size or organisms in the aquatic environment with global warming is a fact (e.g., Daufresne et al. 2009), while the question of how this might affect entire communities is not trivial to resolve (Ohlberger 2013). REFERENCES Angilletta Jr, M. J., T. D. Steury & M. W. Sears, 2004. Temperature, growth rate, and body size in ectotherms: fitting pieces of a life–history puzzle. Integrative and Comparative Biology 44:498-509. https://doi.org/10.1093/icb/44.6.498 Angilletta, M. J. & A. E. Dunham, 2003. The temperature-size rule in ectotherms: Simple evolutionary explanations may not be general. American Naturalist 162(3):332-342. https://doi.org/10.1086/377187 Atkinson, D., 1994. Temperature and organism size – a biological law for ectotherms. Advances in Ecological Research 25:1-58. https://doi.org/10.1016/S0065-2504(08)60212-3 Atkinson, D., S. A. Morley & R. N. Hughes, 2006. From cells to colonies: at what levels of body organization does the 'temperature-size rule' apply? Evolution & Development 8(2):202-214 https://doi.org/10.1111/j.1525-142X.2006.00090.x Audzijonyte, A., D. R. Barneche, A. R. Baudron, J. Belmaker, T. D. Clark, C. T. Marshall, J. R. Morrongiello & I. van Rijn, 2019. Is oxygen limitation in warming waters a valid mechanism to explain decreased body sizes in aquatic ectotherms? Global Ecology and Biogeography 28(2):64-77 https://doi.org/10.1111/geb.12847 Bazin, S., Hemmer-Brepson, C., Logez, M., Sentis, A. & Daufresne, M. 2023. Distinct impacts of food restriction and warming on life history traits affect population fitness in vertebrate ectotherms. HAL, ver.3 peer-reviewed and recommended by PCI Ecology. https://hal.inrae.fr/hal-03738584v3 Berrigan, D. & E. L. Charnov, 1994. Reaction norms for age and size at maturity in response to temperature – a puzzle for life historians. Oikos 70:474-478. https://doi.org/10.2307/3545787 Daufresne, M., K. Lengfellner & U. Sommer, 2009. Global warming benefits the small in aquatic ecosystems. Proceedings of the National Academy of Sciences USA 106(31):12788-93 https://doi.org/10.1073/pnas.0902080106 Forster, J., A. G. Hirst & D. Atkinson, 2012. Warming-induced reductions in body size are greater in aquatic than terrestrial species. Proceedings of the National Academy of Sciences of the United States of America 109(47):19310-19314. https://doi.org/10.1073/pnas.1210460109 Hessen, D. O., P. D. Jeyasingh, M. Neiman & L. J. Weider, 2010. Genome streamlining and the elemental costs of growth. Trends in Ecology & Evolution 25(2):75-80. https://doi.org/10.1016/j.tree.2009.08.004 Kozłowski, J., M. Czarnoleski & M. Dańko, 2004. Can optimal resource allocation models explain why ectotherms grow larger in cold? Integrative and Comparative Biology 44(6):480-493. https://doi.org/10.1093/icb/44.6.480 Leiva, F. P., P. Calosi & W. C. E. P. Verberk, 2019. Scaling of thermal tolerance with body mass and genome size in ectotherms: a comparison between water- and air-breathers. Philosophical Transactions of the Royal Society B 374:20190035. https://doi.org/10.1098/rstb.2019.0035 Ohlberger, J., 2013. Climate warming and ectotherm body szie - from individual physiology to community ecology. Functional Ecology 27:991-1001. https://doi.org/10.1111/1365-2435.12098 Verberk, W. C. E. P., D. Atkinson, K. N. Hoefnagel, A. G. Hirst, C. R. Horne & H. Siepel, 2021. Shrinking body sizes in response to warming: explanations for the temperature-size rule with special emphasis on the role of oxygen. Biological Reviews 96:247-268. https://doi.org/10.1111/brv.12653 Woods, H. A., 1999. Egg-mass size and cell size: effects of temperature on oxygen distribution. American Zoologist 39:244-252. https://doi.org/10.1093/icb/39.2.244 | Distinct impacts of food restriction and warming on life history traits affect population fitness in vertebrate ectotherms | Simon Bazin, Claire Hemmer-Brepson, Maxime Logez, Arnaud Sentis, Martin Daufresne | <p>The reduction of body size with warming has been proposed as the third universal response to global warming, besides geographical and phenological shifts. Observed body size shifts in ectotherms are mostly attributed to the temperature size rul... |  | Climate change, Experimental ecology, Freshwater ecology, Phenotypic plasticity, Population ecology | Aleksandra Walczyńska | 2022-07-27 09:28:29 | View | |
03 Apr 2020
Body temperatures, life history, and skeletal morphology in the nine-banded armadillo (Dasypus novemcinctus)Frank Knight, Cristin Connor, Ramji Venkataramanan, Robert J. Asher https://doi.org/10.17863/CAM.50971Is vertebral count in mammals influenced by developmental temperature? A study with Dasypus novemcinctusRecommended by Mar Sobral based on reviews by Darin Croft and ?Mammals show a very low level of variation in vertebral count, both among and within species, in comparison to other vertebrates [1]. Jordan’s rule for fishes states that the vertebral number among species increases with latitude, due to ambient temperatures during development [2]. Temperature has also been shown to influence vertebral count within species in fish [3], amphibians [4], and birds [5]. However, in mammals the count appears to be constrained, on the one hand, by a possible relationship between the development of the skeleton and the proliferations of cell lines with associated costs (neural malformations, cancer etc., [6]), and on the other by the cervical origin of the diaphragm [7]. References [1] Hautier L, Weisbecker V, Sánchez-Villagra MR, Goswami A, Asher RJ (2010) Skeletal development in sloths and the evolution of mammalian vertebral patterning. Proceedings of the National Academy of Sciences, 107, 18903–18908. doi: 10.1073/pnas.1010335107 | Body temperatures, life history, and skeletal morphology in the nine-banded armadillo (Dasypus novemcinctus) | Frank Knight, Cristin Connor, Ramji Venkataramanan, Robert J. Asher | <p>The nine banded armadillo (*Dasypus novemcinctus*) is the only xenarthran mammal to have naturally expanded its range into the middle latitudes of the USA. It is not known to hibernate, but has been associated with unusually labile core body te... |  | Behaviour & Ethology, Evolutionary ecology, Life history, Physiology, Zoology | Mar Sobral | 2019-11-22 22:57:31 | View | |
29 Dec 2018
The return of the trophic chain: fundamental vs realized interactions in a simple arthropod food webInmaculada Torres-Campos, Sara Magalhães, Jordi Moya-Laraño, Marta Montserrat https://doi.org/10.1101/324178From deserts to avocado orchards - understanding realized trophic interactions in communitiesRecommended by Francis John Burdon based on reviews by Owen Petchey and 2 anonymous reviewersThe late eminent ecologist Gary Polis once stated that “most catalogued food-webs are oversimplified caricatures of actual communities” and are “grossly incomplete representations of communities in terms of both diversity and trophic connections.” Not content with that damning indictment, he went further by railing that “theorists are trying to explain phenomena that do not exist” [1]. The latter critique might have been push back for Robert May´s ground-breaking but ultimately flawed research on the relationship between food-web complexity and stability [2]. Polis was a brilliant ecologist, and his thinking was clearly influenced by his experiences researching desert food webs. Those food webs possess an uncommon combination of properties, such as frequent omnivory, cannibalism, and looping; high linkage density (L/S); and a nearly complete absence of apex consumers, since few species completely lack predators or parasites [3]. During my PhD studies, I was lucky enough to visit Joshua Tree National Park on the way to a conference in New England, and I could immediately see the problems posed by desert ecosystems. At the time, I was ruminating on the “harsh-benign” hypothesis [4], which predicts that the relative importance of abiotic and biotic forces should vary with changes in local environmental conditions (from harsh to benign). Specifically, in more “harsh” environments, abiotic factors should determine community composition whilst weakening the influence of biotic interactions. However, in the harsh desert environment I saw first-hand evidence that species interactions were not diminished; if anything, they were strengthened. Teddy-bear chollas possessed murderously sharp defenses to protect precious water, creosote bushes engaged in belowground “chemical warfare” (allelopathy) to deter potential competitors, and rampant cannibalism amongst scorpions drove temporal and spatial ontogenetic niche partitioning. Life in the desert was hard, but you couldn´t expect your competition to go easy on you. References [1] Polis, G. A. (1991). Complex trophic interactions in deserts: an empirical critique of food-web theory. The American Naturalist, 138(1), 123-155. doi: 10.1086/285208 | The return of the trophic chain: fundamental vs realized interactions in a simple arthropod food web | Inmaculada Torres-Campos, Sara Magalhães, Jordi Moya-Laraño, Marta Montserrat | <p>The mathematical theory describing small assemblages of interacting species (community modules or motifs) has proved to be essential in understanding the emergent properties of ecological communities. These models use differential equations to ... |  | Community ecology, Experimental ecology | Francis John Burdon | 2018-05-16 19:34:10 | View | |
06 Mar 2020

The persistence in time of distributional patterns in marine megafauna impacts zonal conservation strategiesCharlotte Lambert, Ghislain Dorémus, Vincent Ridoux https://doi.org/10.1101/790634The importance of spatio-temporal dynamics on MPA's designRecommended by Sergio Estay based on reviews by Ana S. L. Rodrigues and 1 anonymous reviewerMarine protected areas (MPA) have arisen as the main approach for conservation of marine species. Fishes, marine mammals and birds can be conservation targets that justify the implementation of these areas. However, MPAs undergo many of the problems faced by their terrestrial equivalent. One of the major concerns is that these conservation areas are spatially constrained, by logistic reasons, and many times these constraints caused that key areas for the species (reproductive sites, refugees, migration) fall outside the limits, making conservation efforts even more difficult. Lambert et al. [1] evaluate at what point the Bay of Biscay MPA contains key ecological areas for several emblematic species. The evaluation incorporated a spatio-temporal dimension. To evaluate these ideas, authors evaluate two population descriptors: aggregation and persistence of several species of cetaceans and seabirds. References [1] Lambert, C., Dorémus, G. and V. Ridoux (2020) The persistence in time of distributional patterns in marine megafauna impacts zonal conservation strategies. bioRxiv, 790634, ver. 3 peer-reviewed and recommended by PCI Ecology. doi: 10.1101/790634 | The persistence in time of distributional patterns in marine megafauna impacts zonal conservation strategies | Charlotte Lambert, Ghislain Dorémus, Vincent Ridoux | <p>The main type of zonal conservation approaches corresponds to Marine Protected Areas (MPAs), which are spatially defined and generally static entities aiming at the protection of some target populations by the implementation of a management pla... |  | Conservation biology, Habitat selection, Species distributions | Sergio Estay | 2019-10-03 08:47:17 | View | |
08 Jan 2020

Studies of NH4+ and NO3- uptake ability of subalpine plants and resource-use strategy identified by their functional traitsLegay Nicolas, Grassein Fabrice, Arnoldi Cindy, Segura Raphaël, Laîné Philippe, Lavorel Sandra, Clément Jean-Christophe https://doi.org/10.1101/372235Nitrate or not nitrate. That is the questionRecommended by Sébastien Barot based on reviews by Vincent Maire and 1 anonymous reviewerThe article by Legay et al. [1] addresses two main issues: the links between belowground and aboveground plant traits and the links between plant strategies (as defined by these traits) and the capacity to absorb nitrate and ammonium. I recommend this work because these are important and current issues. The literature on plant traits is extremely rich and the existence of a leaf economic spectrum linked to a gradient between conservative and acquisitive plants is now extremely well established [2-3]. Many teams are now working on belowground traits and possible links with the aboveground gradients [4-5]. It seems indeed that there is a root economic spectrum but this spectrum is apparently less pronounced than the leaf economic spectrum. The existence of links between the two spectrums are still controversial and are likely not universal as suggested by discrepant results and after all a plant could have a conservative strategy aboveground and an acquisitive strategy belowground (or vice-versa) because, indeed, constraints are different belowground and aboveground (for example because in given ecosystem/vegetation type light may be abundant but not water or mineral nutrients). The various results obtained also suggest that we do not full understand the diversity of belowground strategies, what is at stake with these strategies, and the links with root characteristics. References [1] Legay, N., Grassein, F., Arnoldi, C., Segura, R., Laîné, P., Lavorel, S. and Clément, J.-C. (2020). Studies of NH4+ and NO3- uptake ability of subalpine plants and resource-use strategy identified by their functional traits. bioRxiv, 372235, ver. 4 peer-reviewed and recommended by PCI Ecology. doi: 10.1101/372235 | Studies of NH4+ and NO3- uptake ability of subalpine plants and resource-use strategy identified by their functional traits | Legay Nicolas, Grassein Fabrice, Arnoldi Cindy, Segura Raphaël, Laîné Philippe, Lavorel Sandra, Clément Jean-Christophe | <p>The leaf economics spectrum (LES) is based on a suite of leaf traits related to plant functioning and ranges from resource-conservative to resource-acquisitive strategies. However, the relationships with root traits, and the associated belowgro... |  | Community ecology, Physiology, Terrestrial ecology | Sébastien Barot | 2018-07-19 14:22:28 | View | |
29 Mar 2021

Temperature predicts the maximum tree-species richness and water and frost shape the residual variationRicardo A. Segovia https://doi.org/10.1101/836338New light on the baseline importance of temperature for the origin of geographic species richness gradientsRecommended by Joaquín Hortal based on reviews by Rafael Molina-Venegas and 2 anonymous reviewersWhether environmental conditions –in particular energy and water availability– are sufficient to account for species richness gradients (e.g. Currie 1991), or the effects of other biotic and historical or regional factors need to be considered as well (e.g. Ricklefs 1987), was the subject of debate during the 1990s and 2000s (e.g. Francis & Currie 2003; Hawkins et al. 2003, 2006; Currie et al. 2004; Ricklefs 2004). The metabolic theory of ecology (Brown et al. 2004) provided a solid and well-rooted theoretical support for the preponderance of energy as the main driver for richness variations. As any good piece of theory, it provided testable predictions about the sign and shape (i.e. slope) of the relationship between temperature –a key aspect of ambient energy– and species richness. However, these predictions were not supported by empirical evaluations (e.g. Kreft & Jetz 2007; Algar et al. 2007; Hawkins et al. 2007a), as the effects of a myriad of other environmental gradients, regional factors and evolutionary processes result in a wide variety of richness–temperature responses across different groups and regions (Hawkins et al. 2007b; Hortal et al. 2008). So, in a textbook example of how good theoretical work helps advancing science even if proves to be (partially) wrong, the evaluation of this aspect of the metabolic theory of ecology led to current understanding that, while species richness does respond to current climatic conditions, many other ecological, evolutionary and historical factors do modify such response across scales (see, e.g., Ricklefs 2008; Hawkins 2008; D’Amen et al. 2017). And the kinetic model linking mean annual temperature and species richness (Allen et al. 2002; Brown et al. 2004) was put aside as being, perhaps, another piece of the puzzle of the origin of current diversity gradients. Segovia (2021) puts together an elegant way of reinvigorating this part of the metabolic theory of ecology. He uses quantile regressions to model just the upper parts of the relationship between species richness and mean annual temperature, rather than modelling its central tendency through the classical linear regression family of methods –as was done in the past. This assumes that the baseline effect of ambient energy does produce the negative linear relationship between richness and temperature predicted by the kinetic model (Allen et al. 2002), but also that this effect only poses an upper limit for species richness, and the effects of other factors may result in lower levels of species co-occurrence, thus producing a triangular rather than linear relationship. The results of Segovia’s simple and elegant analytical design show unequivocally that the predictions of the kinetic model become progressively more explanatory towards the upper quartiles of the relationship between species richness and temperature along over 10,000 tree local inventories throughout the Americas, reaching over 70% of explanatory power for the upper 5% of the relationship (i.e. the 95% quantile). This confirms to a large extent his reformulation of the predictions of the kinetic model. Further, the neat study from Segovia (2021) also provides evidence confirming that the well-known spatial non-stationarity in the richness–temperature relationship (see Cassemiro et al. 2007) also applies to its upper-bound segment. Both the explanatory power and the slope of the relationship in the 95% upper quantile vary widely between biomes, reaching values similar to the predictions of the kinetic model only in cold temperate environments –precisely where temperature becomes more important than water availability as a constrain to plant life (O’Brien 1998; Hawkins et al. 2003). Part of these variations are indeed related with changes in water deficit and number of frost days along the XXth Century, as shown by the residuals of this paper (Segovia 2021) and a more detailed separate study (Segovia et al. 2020). This pinpoints the importance of the relative balance between water and energy as two of the main climatic factors constraining species diversity gradients, confirming the value of hypotheses that date back to Humboldt’s work (see Hawkins 2001, 2008). There is however a significant amount of unexplained variation in Segovia’s analyses, in particular in the progressive departure of the predictions of the kinetic model as we move towards the tropics, or downwards along the lower quantiles of the richness–temperature relationship. This calls for a deeper exploration of the factors that modify the baseline relationship between richness and energy, opening a new avenue for the macroecological investigation of how different forces and processes shape up geographical diversity gradients beyond the mere energetic constrains imposed by the basal limitations of multicellular life on Earth. References Algar, A.C., Kerr, J.T. and Currie, D.J. (2007) A test of Metabolic Theory as the mechanism underlying broad-scale species-richness gradients. Global Ecology and Biogeography, 16, 170-178. doi: https://doi.org/10.1111/j.1466-8238.2006.00275.x Allen, A.P., Brown, J.H. and Gillooly, J.F. (2002) Global biodiversity, biochemical kinetics, and the energetic-equivalence rule. Science, 297, 1545-1548. doi: https://doi.org/10.1126/science.1072380 Brown, J.H., Gillooly, J.F., Allen, A.P., Savage, V.M. and West, G.B. (2004) Toward a metabolic theory of ecology. Ecology, 85, 1771-1789. doi: https://doi.org/10.1890/03-9000 Cassemiro, F.A.d.S., Barreto, B.d.S., Rangel, T.F.L.V.B. and Diniz-Filho, J.A.F. (2007) Non-stationarity, diversity gradients and the metabolic theory of ecology. Global Ecology and Biogeography, 16, 820-822. doi: https://doi.org/10.1111/j.1466-8238.2007.00332.x Currie, D.J. (1991) Energy and large-scale patterns of animal- and plant-species richness. The American Naturalist, 137, 27-49. doi: https://doi.org/10.1086/285144 Currie, D.J., Mittelbach, G.G., Cornell, H.V., Field, R., Guegan, J.-F., Hawkins, B.A., Kaufman, D.M., Kerr, J.T., Oberdorff, T., O'Brien, E. and Turner, J.R.G. (2004) Predictions and tests of climate-based hypotheses of broad-scale variation in taxonomic richness. Ecology Letters, 7, 1121-1134. doi: https://doi.org/10.1111/j.1461-0248.2004.00671.x D'Amen, M., Rahbek, C., Zimmermann, N.E. and Guisan, A. (2017) Spatial predictions at the community level: from current approaches to future frameworks. Biological Reviews, 92, 169-187. doi: https://doi.org/10.1111/brv.12222 Francis, A.P. and Currie, D.J. (2003) A globally consistent richness-climate relationship for Angiosperms. American Naturalist, 161, 523-536. doi: https://doi.org/10.1086/368223 Hawkins, B.A. (2001) Ecology's oldest pattern? Trends in Ecology & Evolution, 16, 470. doi: https://doi.org/10.1016/S0169-5347(01)02197-8 Hawkins, B.A. (2008) Recent progress toward understanding the global diversity gradient. IBS Newsletter, 6.1, 5-8. https://escholarship.org/uc/item/8sr2k1dd Hawkins, B.A., Field, R., Cornell, H.V., Currie, D.J., Guégan, J.-F., Kaufman, D.M., Kerr, J.T., Mittelbach, G.G., Oberdorff, T., O'Brien, E., Porter, E.E. and Turner, J.R.G. (2003) Energy, water, and broad-scale geographic patterns of species richness. Ecology, 84, 3105-3117. doi: https://doi.org/10.1890/03-8006 Hawkins, B.A., Diniz-Filho, J.A.F., Jaramillo, C.A. and Soeller, S.A. (2006) Post-Eocene climate change, niche conservatism, and the latitudinal diversity gradient of New World birds. Journal of Biogeography, 33, 770-780. doi: https://doi.org/10.1111/j.1365-2699.2006.01452.x Hawkins, B.A., Albuquerque, F.S., Araújo, M.B., Beck, J., Bini, L.M., Cabrero-Sañudo, F.J., Castro Parga, I., Diniz-Filho, J.A.F., Ferrer-Castán, D., Field, R., Gómez, J.F., Hortal, J., Kerr, J.T., Kitching, I.J., León-Cortés, J.L., et al. (2007a) A global evaluation of metabolic theory as an explanation for terrestrial species richness gradients. Ecology, 88, 1877-1888. doi:10.1890/06-1444.1. doi: https://doi.org/10.1890/06-1444.1 Hawkins, B.A., Diniz-Filho, J.A.F., Bini, L.M., Araújo, M.B., Field, R., Hortal, J., Kerr, J.T., Rahbek, C., Rodríguez, M.Á. and Sanders, N.J. (2007b) Metabolic theory and diversity gradients: Where do we go from here? Ecology, 88, 1898–1902. doi: https://doi.org/10.1890/06-2141.1 Hortal, J., Rodríguez, J., Nieto-Díaz, M. and Lobo, J.M. (2008) Regional and environmental effects on the species richness of mammal assemblages. Journal of Biogeography, 35, 1202–1214. doi: https://doi.org/10.1111/j.1365-2699.2007.01850.x Kreft, H. and Jetz, W. (2007) Global patterns and determinants of vascular plant diversity. Proceedings of the National Academy of Sciences USA, 104, 5925-5930. doi: https://doi.org/10.1073/pnas.0608361104 O'Brien, E. (1998) Water-energy dynamics, climate, and prediction of woody plant species richness: an interim general model. Journal of Biogeography, 25, 379-398. doi: https://doi.org/10.1046/j.1365-2699.1998.252166.x Ricklefs, R.E. (1987) Community diversity: Relative roles of local and regional processes. Science, 235, 167-171. doi: https://doi.org/10.1126/science.235.4785.167 Ricklefs, R.E. (2004) A comprehensive framework for global patterns in biodiversity. Ecology Letters, 7, 1-15. doi: https://doi.org/10.1046/j.1461-0248.2003.00554.x Ricklefs, R.E. (2008) Disintegration of the ecological community. American Naturalist, 172, 741-750. doi: https://doi.org/10.1086/593002 Segovia, R.A. (2021) Temperature predicts the maximum tree-species richness and water and frost shape the residual variation. bioRxiv, 836338, ver. 4 peer-reviewed and recommended by Peer community in Ecology. doi: https://doi.org/10.1101/836338 Segovia, R.A., Pennington, R.T., Baker, T.R., Coelho de Souza, F., Neves, D.M., Davis, C.C., Armesto, J.J., Olivera-Filho, A.T. and Dexter, K.G. (2020) Freezing and water availability structure the evolutionary diversity of trees across the Americas. Science Advances, 6, eaaz5373. doi: https://doi.org/10.1126/sciadv.aaz5373 | Temperature predicts the maximum tree-species richness and water and frost shape the residual variation | Ricardo A. Segovia | <p>The kinetic hypothesis of biodiversity proposes that temperature is the main driver of variation in species richness, given its exponential effect on biological activity and, potentially, on rates of diversification. However, limited support fo... |  | Biodiversity, Biogeography, Botany, Macroecology, Species distributions | Joaquín Hortal | 2019-11-10 20:56:40 | View | |
18 Apr 2024

Insights on the effect of mega-carcass abundance on the population dynamics of a facultative scavenger predator and its preyMellina Sidous; Sarah Cubaynes; Olivier Gimenez; Nolwenn Drouet-Hoguet; Stephane Dray; Loic Bollache; Daphine Madhlamoto; Nobesuthu Adelaide Ngwenya; Herve Fritz; Marion Valeix https://doi.org/10.1101/2023.11.08.566247Unveiling the influence of carrion pulses on predator-prey dynamicsRecommended by Esther Sebastián González based on reviews by Eli Strauss and 1 anonymous reviewer based on reviews by Eli Strauss and 1 anonymous reviewer
Most, if not all, predators consume carrion in some circumstances (Sebastián-Gonzalez et al. 2023). Consequently, significant fluctuations in carrion availability can impact predator-prey dynamics by altering the ratio of carrion to live prey in the predators' diet (Roth 2003). Changes in carrion availability may lead to reduced predation when carrion is more abundant (hypo-predation) and intensified predation if predator populations surge in response to carrion influxes but subsequently face scarcity (hyper-predation), (Moleón et al. 2014, Mellard et al. 2021). However, this relationship between predation and scavenging is often challenging because of the lack of empirical data. | Insights on the effect of mega-carcass abundance on the population dynamics of a facultative scavenger predator and its prey | Mellina Sidous; Sarah Cubaynes; Olivier Gimenez; Nolwenn Drouet-Hoguet; Stephane Dray; Loic Bollache; Daphine Madhlamoto; Nobesuthu Adelaide Ngwenya; Herve Fritz; Marion Valeix | <p>The interplay between facultative scavenging and predation has gained interest in the last decade. The prevalence of scavenging induced by the availability of large carcasses may modify predator density or behaviour, potentially affecting prey.... |  | Community ecology | Esther Sebastián González | Eli Strauss | 2023-11-14 15:27:16 | View |
10 Aug 2023
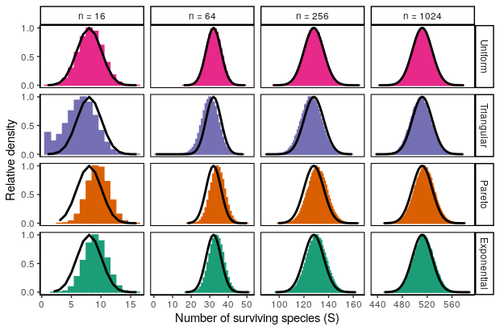
Coexistence of many species under a random competition-colonization trade-offZachary R. Miller, Maxime Clenet, Katja Della Libera, François Massol, Stefano Allesina https://doi.org/10.1101/2023.03.23.533867Assembly in metacommunities driven by a competition-colonization tradeoff: more species in, more species outRecommended by Frederik De Laender based on reviews by Canan Karakoç and 1 anonymous reviewerThe output of a community model depends on how you set its parameters. Thus, analyses of specific parameter settings hardwire the results to specific ecological scenarios. Because more general answers are often of interest, one tradition is to give models a statistical treatment: one summarizes how model parameters vary across species, and then predicts how changing the summary, instead of the individual parameters themselves, would change model output. Arguably the best-known example is the work initiated by May, showing that the properties of a community matrix, encoding effects species have on each other near their equilibrium, determine stability (1,2). More recently, this statistical treatment has also been applied to one of community ecology’s more prickly and slippery subjects: community assembly, which deals with the question “Given some regional species pool, which species will be able to persist together at some local ecosystem?”. Summaries of how species grow and interact in this regional pool predict the fraction of survivors and their relative abundances, the kind of dynamics, and various kinds of stability (3,4). One common characteristic of such statistical treatments is the assumption of disorder: if species do not interact in too structured ways, simple and therefore powerful predictions ensue that often stand up to scrutiny in relatively ordered systems. 2. Allesina, S. & Tang, S. (2015). The stability–complexity relationship at age 40: a random matrix perspective. Population Ecology, 57, 63–75. https://doi.org/10.1007/s10144-014-0471-0 3. Bunin, G. (2016). Interaction patterns and diversity in assembled ecological communities. Preprint at http://arxiv.org/abs/1607.04734. 5. Miller, Z. R., Clenet, M., Libera, K. D., Massol, F. & Allesina, S. (2023). Coexistence of many species under a random competition-colonization trade-off. bioRxiv 2023.03.23.533867, ver 3 peer-reviewed and recommended by PCI Ecology. https://doi.org/10.1101/2023.03.23.533867 6. Serván, C. A. & Allesina, S. (2021). Tractable models of ecological assembly. Ecology Letters, 24, 1029–1037. https://doi.org/10.1111/ele.13702 | Coexistence of many species under a random competition-colonization trade-off | Zachary R. Miller, Maxime Clenet, Katja Della Libera, François Massol, Stefano Allesina | <p>The competition-colonization trade-off is a well-studied coexistence mechanism for metacommunities. In this setting, it is believed that coexistence of all species requires their traits to satisfy restrictive conditions limiting their similarit... |  | Biodiversity, Coexistence, Colonization, Community ecology, Competition, Population ecology, Spatial ecology, Metacommunities & Metapopulations, Theoretical ecology | Frederik De Laender | 2023-03-30 20:42:48 | View |
MANAGING BOARD
Julia Astegiano
Tim Coulson
Anna Eklof
Dominique Gravel
François Massol
Ben Phillips
Cyrille Violle










