
Effect of food conditions on the Temperature-Size Rule
Distinct impacts of food restriction and warming on life history traits affect population fitness in vertebrate ectotherms
Abstract
Recommendation: posted 17 May 2023, validated 17 May 2023
Walczyńska, A. (2023) Effect of food conditions on the Temperature-Size Rule. Peer Community in Ecology, 100464. https://doi.org/10.24072/pci.ecology.100464
Recommendation
Temperature-size rule (TSR) is a phenomenon of plastic changes in body size in response to temperature, originally observed in more than 80% of ectothermic organisms representing various groups (Atkinson 1994). In particular, ectotherms were observed to grow faster and reach smaller size at higher temperature and grow slower and achieve larger size at lower temperature. This response has fired the imagination of researchers since its invention, due to its counterintuitive pattern from an evolutionary perspective (Berrigan and Charnov 1994). The main question to be resolved is: why do organisms grow fast and achieve smaller sizes under more favourable conditions (= relatively higher temperature), while they grow longer and achieve larger sizes under less favourable conditions (relatively lower temperature), if larger size means higher fitness, while longer development may be risky?
This evolutionary conundrum still awaits an ultimate explanation (Angilletta Jr et al. 2004; Angilletta and Dunham 2003; Verberk et al. 2021). Although theoretical modelling has shown that such a growth pattern can be achieved as a response to temperature alone, with a specific combination of energetic parameters and external mortality (Kozłowski et al. 2004), it has been suggested that other temperature-dependent environmental variables may be the actual drivers of this pattern. One of the most frequently invoked variable is the relative oxygen availability in the environment (e.g., Atkinson et al. 2006; Audzijonyte et al. 2019; Verberk et al. 2021; Woods 1999), which decreases with temperature increase. Importantly, this effect is more pronounced in aquatic systems (Forster et al. 2012). However, other temperature-dependent parameters are also being examined in the context of their possible effect on TSR induction and strength.
Food availability is among the interfering factors in this regard. In aquatic systems, nutritional conditions are generally better at higher temperature, while a range of relatively mild thermal conditions is considered. However, there are no conclusive results so far on how nutritional conditions affect the plastic body size response to acute temperature changes. A study by Bazin et al. (2023) examined this particular issue, the effects of food and temperature on TSR, in medaka fish. An important value of the study was to relate the patterns found to fitness. This is a rare and highly desirable approach since evolutionary significance of any results cannot be reliably interpreted unless shown as expressed in light of fitness.
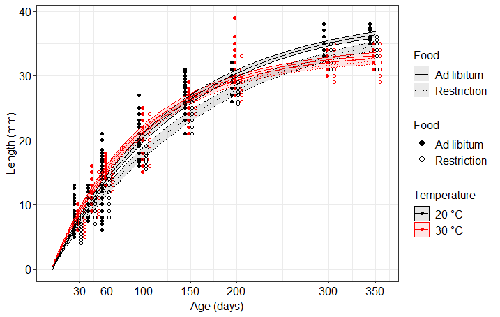
The authors compared the body size of fish kept at 20°C and 30°C under conditions of food abundance or food restriction. The results showed that the TSR (smaller body size at 30°C compared to 20°C) was observed in both food treatments, but the effect was delayed during fish development under food restriction. Regarding the relevance to fitness, increased temperature resulted in more eggs laid but higher mortality, while food restriction increased survival but decreased the number of eggs laid in both thermal treatments. Overall, food restriction seemed to have a more severe effect on development at 20°C than at 30°C, contrary to the authors’ expectations.
I found this result particularly interesting. One possible interpretation, also suggested by the authors, is that the relative oxygen availability, which was not controlled for in this study, could have affected this pattern. According to theoretical predictions confirmed in quite many empirical studies so far, oxygen restriction is more severe at higher temperatures. Perhaps for these particular two thermal treatments and in the case of the particular species studied, this restriction was more severe for organismal performance than the food restriction. This result is an example that all three variables, temperature, food and oxygen, should be taken into account in future studies if the interrelationship between them is to be understood in the context of TSR. It also shows that the reasons for growing smaller in warm may be different from those for growing larger in cold, as suggested, directly or indirectly, in some previous studies (Hessen et al. 2010; Leiva et al. 2019).
Since medaka fish represent predatory vertebrates, the results of the study contribute to the issue of global warming effect on food webs, as the authors rightly point out. This is an important issue because the general decrease in the size or organisms in the aquatic environment with global warming is a fact (e.g., Daufresne et al. 2009), while the question of how this might affect entire communities is not trivial to resolve (Ohlberger 2013).
REFERENCES
Angilletta Jr, M. J., T. D. Steury & M. W. Sears, 2004. Temperature, growth rate, and body size in ectotherms: fitting pieces of a life–history puzzle. Integrative and Comparative Biology 44:498-509. https://doi.org/10.1093/icb/44.6.498
Angilletta, M. J. & A. E. Dunham, 2003. The temperature-size rule in ectotherms: Simple evolutionary explanations may not be general. American Naturalist 162(3):332-342. https://doi.org/10.1086/377187
Atkinson, D., 1994. Temperature and organism size – a biological law for ectotherms. Advances in Ecological Research 25:1-58. https://doi.org/10.1016/S0065-2504(08)60212-3
Atkinson, D., S. A. Morley & R. N. Hughes, 2006. From cells to colonies: at what levels of body organization does the 'temperature-size rule' apply? Evolution & Development 8(2):202-214 https://doi.org/10.1111/j.1525-142X.2006.00090.x
Audzijonyte, A., D. R. Barneche, A. R. Baudron, J. Belmaker, T. D. Clark, C. T. Marshall, J. R. Morrongiello & I. van Rijn, 2019. Is oxygen limitation in warming waters a valid mechanism to explain decreased body sizes in aquatic ectotherms? Global Ecology and Biogeography 28(2):64-77 https://doi.org/10.1111/geb.12847
Bazin, S., Hemmer-Brepson, C., Logez, M., Sentis, A. & Daufresne, M. 2023. Distinct impacts of food restriction and warming on life history traits affect population fitness in vertebrate ectotherms. HAL, ver.3 peer-reviewed and recommended by PCI Ecology. https://hal.inrae.fr/hal-03738584v3
Berrigan, D. & E. L. Charnov, 1994. Reaction norms for age and size at maturity in response to temperature – a puzzle for life historians. Oikos 70:474-478. https://doi.org/10.2307/3545787
Daufresne, M., K. Lengfellner & U. Sommer, 2009. Global warming benefits the small in aquatic ecosystems. Proceedings of the National Academy of Sciences USA 106(31):12788-93 https://doi.org/10.1073/pnas.0902080106
Forster, J., A. G. Hirst & D. Atkinson, 2012. Warming-induced reductions in body size are greater in aquatic than terrestrial species. Proceedings of the National Academy of Sciences of the United States of America 109(47):19310-19314. https://doi.org/10.1073/pnas.1210460109
Hessen, D. O., P. D. Jeyasingh, M. Neiman & L. J. Weider, 2010. Genome streamlining and the elemental costs of growth. Trends in Ecology & Evolution 25(2):75-80. https://doi.org/10.1016/j.tree.2009.08.004
Kozłowski, J., M. Czarnoleski & M. Dańko, 2004. Can optimal resource allocation models explain why ectotherms grow larger in cold? Integrative and Comparative Biology 44(6):480-493. https://doi.org/10.1093/icb/44.6.480
Leiva, F. P., P. Calosi & W. C. E. P. Verberk, 2019. Scaling of thermal tolerance with body mass and genome size in ectotherms: a comparison between water- and air-breathers. Philosophical Transactions of the Royal Society B 374:20190035. https://doi.org/10.1098/rstb.2019.0035
Ohlberger, J., 2013. Climate warming and ectotherm body szie - from individual physiology to community ecology. Functional Ecology 27:991-1001. https://doi.org/10.1111/1365-2435.12098
Verberk, W. C. E. P., D. Atkinson, K. N. Hoefnagel, A. G. Hirst, C. R. Horne & H. Siepel, 2021. Shrinking body sizes in response to warming: explanations for the temperature-size rule with special emphasis on the role of oxygen. Biological Reviews 96:247-268. https://doi.org/10.1111/brv.12653
Woods, H. A., 1999. Egg-mass size and cell size: effects of temperature on oxygen distribution. American Zoologist 39:244-252. https://doi.org/10.1093/icb/39.2.244
The recommender in charge of the evaluation of the article and the reviewers declared that they have no conflict of interest (as defined in the code of conduct of PCI) with the authors or with the content of the article. The authors declared that they comply with the PCI rule of having no financial conflicts of interest in relation to the content of the article.
Evaluation round #2
DOI or URL of the preprint: https://hal.inrae.fr/hal-03738584v2
Version of the preprint: 2
Author's Reply, 09 May 2023
Decision by Aleksandra Walczyńska, posted 02 Jan 2023, validated 02 Jan 2023
Below I provide, first, the summary of referees’ comments to the previous version of the text, and the overview of authors’ responses. Later, I present my own comments to the current version of the text, which justify my decision on the manuscript, which is, to revise.
The comments of Referee 1 can be summarized in the way that the reviewer lacks, firstly, a presentation of the research in a broader context and, secondly, a clear emphasis on what new the research brings to the subject. I appreciate the improvement made by the authors in the first of the indicated contexts. The authors have referred more extensively to the literature related to the subject. However, there is some insufficiency regarding the second point, namely the importance of the results presented in light of the research topic. The authors argue that the most important novelty resulting from the work is that the study was performed on a species that is large and is a predatory vertebrate. I am not convinced that this is a sufficient argument, because in that case one could perform similar studies for each species separately and use the same argument accordingly. Then, all knowledge would be limited to "case studies". What I miss is putting the results interpretation in the right context. I have a suggestion, which the authors could use at their discretion. The presented results could be interpreted in the context of the complexity of body size control in different organisms. To date, similar studies have been done on rotifers, copepods, insects and others. By comparing the results based on organism-specific body size control system during the lifecycle, one can make attempts to summarize the similarities and differences found so far. This topic is actually a very clear and important gap in research on the TSR, raised in discussion in many papers while it is just touched in the presented manuscript (actually, in the last sentence of Abstract). Some other ways of interpretation are of course possible.
Summarizing the comments of Referee 2, the major points referred to discussing the results in the wider evolutionary perspective, e.g., proximate vs. ultimate mechanisms, other limiting factors, size at maturity vs. asymptotic size. The authors made attempts to address these points, but I found them insufficient. The least satisfactory seems to be a reference to size at maturity vs. asymptotic size. I suggest to refer to existing literature in this regard (it is indeed limited, but some information is available). In the detailed comments below I aimed to clarify some issues to facilitate the interpretation of data in the more evolutionary context. Referee 2 made also some comments regarding statistical issues and those I found sufficiently addressed.
Below are my detailed comments to the current version of the text:
L. 30-31 - “food restriction appears to amplify TSR by decreasing initial growth rate in the cold treatment” – as invoked by Referee 2, the strength of TSR generally refers to changes in body size and not in growth rate.
L. 34 – though I strongly applaud referring the results to fitness measures, I am not convinced that the reference to “live fast die young” is appropriate/necessary in the case of this study. In the context of response to higher temperature it is quite trivial, because temperature accelerates the biochemical reactions in living organisms and such a result is the only expected.
L. 56-57 – there are also some, though indeed limited, studies on ultimate mechanisms, like the one by Walczynska et al. 2015 (the one suggested by Referee 2; 10.1016/j.jtherbio.2014.11.002).
L. 63-65 – the TSR is weaker in terrestrial systems but it does not implicate that oxygen is not limiting there, but rather that there is quite many other interfering factors, among which the most important seems to be seasonality (e.g., Verberk et al. 2021).
L – 65-66 – “At the individual level, body size shift can be explained by the impact of temperature on the growth of ectotherms” – I do not understand this sentence. It is either trivial, or an unwanted shortcut.
L. 68 – as invoked by Referee 2, originally TSR was referred to size at maturity and not to asymptotic size.
L. 70-72 – to put it simple, ultimate factors refer to fitness, namely, to evolutionary meaning of the studied phenomenon. The current sentence is too complicated and not exactly to the point.
L. 74-76 – again, TSR refers to body size response, which is of course accompanied by the whole growth and development pattern, but body size remains in the centre of interest.
L. 80-83 – I do not understand the reasoning presented in this sentence.
L. 108-122 – This paragraph should be removed from Introduction, because the consequences of the examined effect for the food web interactions were not the aim of the study. Definitely, it is still an important point for Discussion section.
L. 124-129 – Such effects were also studied in rotifers (Kielbasa et al. 2014; 10.1002/ece3.1292) and in diatoms (Walczyńska and Sobczyk 2017; 10.1002/ece3.3263).
L. 142-146 – In this sentence there is a mixture of what was actually addressed in this study and what could be interpreted out of the results.
L. 150-155 – these lengthy sentences should be shortened to one precise message.
L. 147-160 – again, a mixture of goals and interpretations, while in this section the sound, reliable hypotheses are expected.
L. 171-174 – Referee 1 raised an important point about the thermal range investigated. I found the authors’ response to that comment insufficient. First of all, if the optimal temperature for the species is 25 °C, then 30 °C in a thermal treatment is above the optimum and should rather be considered a suboptimal temperature. I totally understand the logistical limitations which sometimes dictate the choice of treatments in the planned study, but the most important then is to correctly interpret the data. In this particular case, the difference between the two thermal regimes is that at 20 °C the response is assumed to be fully plastic, because it is within the ‘optimal thermal range’ for the TSR (Walczyńska et al. 2016; 10.1016/j.jtherbio.2016.06.006, the work suggested by Referee 1), while at 30 °C, being above this range, some compensatory/alternative physiological mechanisms are expected to be launched. Actually, such a distinction makes the interpretation of the patterns found at each temperature much easier.
L. 192-193 – Does it mean the highest pre-maturity mortality in this regime?
L. 273-279 – I find this interpretation of Fig. 2 quite misleading. In reference to the theory presented in Fig. 1, the comparison of crossed vs. nested patterns should be made for the pairs ad_20 vs. ad_30 and res_20 vs. res_30, because this distinction is expected for comparison across temperature. Then, the effect of food should be compared for the patterns found for both ads vs. both reses.
Figure 2 – age at maturity could be additionally displayed in the figure for comparison.
L. 302-306 – If I am correct, survival was estimated starting from the age at maturity. In that case, it should be stated clearly, here and throughout the manuscript, and I suggest considering the change of the name of this trait to ‘life expectancy since maturity’. Otherwise it is misleading, especially that, referring to my comment above, the highest pre-maturity mortality was found in the res_20 regime.
L. 324-331 – this paragraph resembles very much the one presented in Introduction. Please, choose one part of the text, the one in which it will be more suitable. Also, the reference to stronger TSR response with larger size according to Forster et al. 2012 is generally correct, but it is not an argument in this case. Forster et al. 2012 compared species which are close on a phylogenetic tree. For an obvious reason, it would not be correct to expect that fish species have stronger TSR pattern than, e.g., rotifer species, just because they are larger.
Discussion in general – perhaps there are different “schools” in this regard, but I was trained that discussion should start from presentation of the most important findings from the study in the wider perspective. The speculative part should be following this introductory one.
L. 339-354 – This section should be much more concise, as it is speculative and does not refer directly to the main aim of the study. I appreciate the discussion on the consequences of thermally-induced body size changes in a predatory species for the whole community or a trophic web, but this should be much shorter.
L. 364-368 – Which curves did the authors mean to be nested in this sentence? Please, see my comment above about comparing the crossed vs. nested curves. It is not clear what the authors found surprising.
L. 383-400 – This part is far too long. Information is just repeated using slightly different words. Referring to the oxygen as a limiting factor, one of the most important differences between oxygen and food availabilities is that the former generally decreases with increasing temperature (at least relatively to demands), while the latter generally increases with increasing temperature (in ecologically relevant conditions). Walczyńska and Sobczyk (2017; 10.1002/ece3.3263) discuss the differences in thermally driven nutrition vs. thermally driven oxygen effects on plastic body size response.
L. 420-429 – This paragraph is highly speculative and not really understandable. Instead, one would expect a joint discussion of the experimental effects on different life history traits, namely size at maturity, age at maturity, fecundity, clutch size and survival (life expectancy since maturity?). What about the trade-offs, which are mentioned in Introduction?
L. 431-432 – Again, TSR refers to body size and not to growth rate.
L. 447-449 – This assumption should be definitely more elaborated. My feeling while reading this manuscript was that the food-restriction applied in the study was not a real restriction. The authors should provide some references in which such a way of feeding was proven to be a restriction (through, for example, slower growth rate or smaller final size). Shortly, why this particular way of food regimes distinction was chosen?
L. 472-490 – This paragraph is again too speculative and too wordy. Perhaps it should be matched with the very similar paragraph which was presented at the beginning of Discussion to provide a short, reliable and concise information on where and how these results could be implemented.
Evaluation round #1
DOI or URL of the preprint: https://hal.archives-ouvertes.fr/hal-03738584v1
Author's Reply, 07 Dec 2022
Decision by Aleksandra Walczyńska, posted 26 Sep 2022
Dear Authors,
The two Reviewers point out that the study is potentially interesting and relatively well prepared. However, they also point some important shortcomings regarding either the results interpretation and discussion, or the methodology description. The solutions for text improvement provided by the Reviewers are clear and sound, therefore I suggest you to revise the text accordingly.
With kind regards,
Aleksandra Walczyńska
Reviewed by Wolf Blanckenhorn, 17 Sep 2022
Review PCIEvoBio : ‘Distinct_impacts_of_resource_restriction_and_warming_on_growth_and_survival
This empirical paper subsumes various long-standing life history phenomena (‘rules’) to apply them in the climate change context, most centrally the temperature-size-rule (TSR; i.e. the empirical phenomenon that ectotherms grow to a smaller size at warmer temperatures: Atkinson 1994). A standard laboratory growth experiment was performed on Medaka, an often-used and easy-to-hold small fresh water fish, at 2 temperatures (20C & 30C) & 2 food treatments (abundant & restricted). The authors argue that such interactive effects of food and temperature on animal life history traits (notably body size, but here also growth, development and longevity) are rarely studied, but this is certainly not true in invertebrates (notably insects, where this is almost standard). Regardless, albeit minimalist in terms of the number of treatments (2x2) of each environmental axis, such an experiment was worth performing in this context in a fish for which it has not been performed before. I think the main ‘advance’ -- if there is any, as this is certainly not novel as such -- of this study lies in monitoring the entire non-linear growth of the fish (for ca. 1 year) under the 4 treatment combinations (Fig. 2), and in interpreting the results in terms of plastic vs. evolutionary life history adjustments in the climate change context.
The main result seems to be a significant interaction between temperature and food level, although in the end the (expected) crossing growth trajectories are only evident for temperature (Fig. 1a) and not for food treatment (Fig. 1b), both as expected and well demonstrated before in other species (in this context). As mentioned before, this result is worth publishing for a new species to demonstrate consistency in diversity, but nothing particularly earth-shattering.
The Discussion in the eco-evolutionary life history context is fair and covers all necessary ground by integrating by now classic research from various life history realms (including ageing) in the climate change context, as is fashionable presently. In the end, however, the actual fitness costs & benefits of the various trajectories have not been investigated in a range of realistic environments (beyond assessing final body size and lifespan; e.g. in terms of effects on male mating success or female fecundity). As mentioned above, I would have liked to see more temperature and food levels being tested to assess likely relevant non-linearities. By now it is well known that part of the problem of the TSR are incomplete temperature ranges being assessed (e.g. Walczynska et al. 2016 JThermBio //doi.org/10.1016/j.jtherbio.2016.06.006; Blanckenhorn et al. 2021 JThermBio //doi.org/10.1016/j.jtherbio.2021.103069). So ultimately, the question of whether the TSR is adaptive (or a physiological constraint) and beneficial for the persistence of populations and species in the face of climate change under realistic food limitations could not be answered with this study either (which I wouldn’t have expected anyway). The TSR ‘puzzle’ has not been solved yet. This is merely another (limited) data point.
The entire paper is too focussed on fish and the aquatic realm (e.g. Daphnia; but omitting the work by e.g. Roby Stoks’ lab on odonates) in terms of citations, so the Discussion should expand more (and compare) (in)to the terrestrial realm in general (e.g. Rohner et al. 2017 EvoDevo DOI: 10.1111/ede.12223). This is important because TSR patterns differ in general in aquatic vs. terrestrial taxa (Forster et al. 2012; Hirst & Forster 2013). For these reasons, I would publish this work in a fish or aquatic journal if this is so desired.
Methodologically this paper seems sound (albeit a bit minimalist; see above). What precisely is a replicate in Fig. 2 should be explicitly stated (groups of fish in small tanks vs. individual fish, as mentioned in the Methods on P5, center), as is now not entirely clear. Overall the paper is well presented.
Wolf.Blanckenhorn@uzh.ch, University of Zürich
https://doi.org/10.24072/pci.ecology.100464.rev11Reviewed by Wilco Verberk, 10 Sep 2022
In their manuscript, Bazin et al. investigate how food quantity modulates thermal effects on survival, growth and the resulting body size. I found this to be an interesting study with valuable insights and have provided some comments and suggestions below which I hope the authors will find helpful in improving their manuscript.
Introduction:
In general, the introduction reads well and explains the main differences in how growth performance responds to either temperature or food quantity (e.g. in fig 1). However, I missed a deeper introduction on the difference between proximate mechanisms (which tend to be rooted in physiology) and ultimate mechanisms (which tend to take an evolutionary perspective grounded in fitness). This study investigates both responses in growth (which could be argued to reflect more proximate mechanisms) and survival (which could be argued to be more ultimate). I believe there is scope for integrating both approaches, possibly deriving expectations on the interactive effect of food restriction (increasing survival and hence selecting for late maturation at a larger size) and warming (decreasing survival and hence selecting for early maturation).
Line 47: Several earlier studies that have studied the underlying proximate mechanisms have focused on resource limitation in warm conditions, constraining growth rate later in ontogeny. These have mostly focused on oxygen as a limiting resource rather than food and found that T-S responses tend to be amplified under hypoxia, i.e. when recources are more likely to be limiting (Frazier et al., 2001; Hoefnagel & Verberk, 2015). It may be worthwhile to point out if and how responses to growth as a limiting resource are similar or different from oxygen as a limiting resource. Studies on rotifers have also found support for oxygen limitation as an ultimate explanation (Walczynska et al., 2015). Related to this, I would suggest to replace resource with food throughout the manuscript whenever resource pertains to food (e.g. change resource restriction to food restriction). See also e.g. lines 222, 226, 247.
Line 52: The TSR is not exclusively defined by asymptotic size, but is also frequently evaluated by size at maturity (which is different from asymptotic size at least in species with indeterminate growth, such as fish). It may be good to emphasize that the TSR is about comparing body size at a comparable life stage.
Methods
Line 128: Given that around 80 fish were monitored per treatment and aquaria held 20-30 fish, does that mean that you had around 4 aquaria replicates for each treatment? If so, did you include tank replicate as a random factor?
Line 143: indentify should read identified.
Line 176: Just out of curiosity: was there an autocorrelation between the estimated parameter value for K and Linfinity within a given treatment? My experience with model fitting is that this can sometimes happen: faster growth to a larger asymptotic size can yield a very similar growth cure (plotting length vs time) as slower growth to a smaller size.
Results:
Line 190: When are fish adults? In the methods it is stated that they reach maturity after 10-12 weeks at 25C. Presumably, they reach maturity earlier at 30C and later at 20C. If you have information on how temperature and food restriction affected age at maturity, I would suggest to include it so that the size at this age can be compared. Without this information, what is left is comparing asymptotic size, but from the wording and the graph it is unclear how asymptotic size responds. This information is presented in Fig S2, but not referred to in the results.
Discussion
In general, I found the discussion to be somewhat long. I think there is possibilities to reduce it somewhat and also restructure it. Maybe focusing first on growth trajectories (why does food restriction reduce growth more in the cold treatment), then survival, followed by their integration (can we understand responses in size at maturity/asymptotic best from effects of temperature and food on growth or from their effects on survival?), ending with a brief paragraph on implications (for trophic relationships and how to model these).
Line 221: replace Although with Because
Line 230: See comment about the difference between adult and asymptotic size above.
Line 240: I think a recent paper does consider temperature effects of body size in a foodweb context (Lindmark et al., 2022).
Line 246: Note that a recent study showed that effects of mass and temperature on metabolism are interacting such that the effect of temperature varies between large and small fish (Rubalcaba et al., 2020).
Line 259: If growth rates are more limited in warm than in cold, I do not see how the results for Amphiprion larvae are similar.
Line 261: I feel there is more that could be said here? Do you mean that at lower temperatures the stoichiometric ratio’s needed change in such a way that food restriction has more severe effects?
Line 274: I urge the authors to include this unpublished data and use the temperature (and food treatment) specific ages at maturity to make a comparison for size at maturity and whether thermal responses in size at maturity are magnified under food restriction.
Line 280: I presume that food intake was not quantified precisely. Still I wonder if fish exhibited compensatory feeding such that on a feeding day the restricted fish had greater appetite and ate more? Related to this, does this fish species have a stomach which it can use to ‘overfeed’ and store food?
Line 281,305: I think the result of a stronger TSR needs to be better argued/supported in the results. Currently, the differences in asymptotic size (Fig S2) are perhaps slightly more pronounced under food restriction, but the large CI do not make this a convincing argument for a stronger TSR. If the argument is instead based on the stronger reduction in initial growth following food restriction in the cold, then this has to be better explained: Most researchers would evaluate the strength of the TSR based on size alone, not necessarily the growth trajectory.
Line 282: Not sure how relevant the work on unicellular organisms is for the fish as the constraints for uptake of resources are quite different.
Line 293: Note that this review appeared in print in 2021, not 2020.
References:
Frazier M.R., Woods H.A. & Harrison J.F. (2001). Interactive effects of rearing temperature and oxygen on the development of Drosophila melanogaster. Physiological and Biochemical Zoology 74, 641–50.
Hoefnagel, K.N. & Verberk, W.C.E.P. (2015). Is the temperature-size rule mediated by oxygen in aquatic ectotherms? Journal of Thermal Biology 54, 56–65.
Lindmark, M., Audzijonyte, A., Blanchard, J. L., & Gårdmark, A. (2022). Temperature impacts on fish physiology and resource abundance lead to faster growth but smaller fish sizes and yields under warming. Global Change Biology, 00, 1– 15. https://doi.org/10.1111/gcb.16341
Rubalcaba JG, Verberk WCEP, Hendriks AJ, Saris B & Woods HA (2020) Oxygen limitation may affect the temperature and size dependence of metabolism in aquatic ectotherms. Proceedings of the National Academy of Sciences of the USA, 117:31963-31968
Walczyńska, A., Labecka A.M., Sobczyk, M., Czarnołęski, M. & Kozłowski, J. (2015). The Temperature–Size Rule in Lecane inermis (Rotifera) is adaptive and driven by nuclei size adjustment to temperature and oxygen combinations. Journal of Thermal Biology 54, 78–85.
https://doi.org/10.24072/pci.ecology.100464.rev12










