Latest recommendations

| Id | Title▲ | Authors | Abstract | Picture | Thematic fields | Recommender | Reviewers | Submission date | |
|---|---|---|---|---|---|---|---|---|---|
25 May 2021

Clumpy coexistence in phytoplankton: The role of functional similarity in community assemblyCaio Graco-Roza, Angel M. Segura, Carla Kruk, Patricia Domingos, Janne Soininen, Marcelo M. Marinho https://doi.org/10.1101/869966Environmental heterogeneity drives phytoplankton community assembly patterns in a tropical riverine systemRecommended by Cédric Hubas and Eric Goberville and Eric Goberville based on reviews by Eric Goberville and Dominique Lamy based on reviews by Eric Goberville and Dominique Lamy
What predisposes two individuals to form and maintain a relationship is a fundamental question. Using facial recognition to see whether couples' faces change over time to become more and more similar, psychology researchers have concluded that couples tend to be formed from the start between people whose faces are more similar than average [1]. As the saying goes, birds of a feather flock together. And what about in nature? Are these rules of assembly valid for communities of different species? In his seminal contribution, Robert MacArthur (1984) wrote ‘To do science is to search for repeated patterns’ [2]. Identifying the mechanisms that govern the arrangement of life is a hot research topic in the field of ecology for decades, and an absolutely essential prerequisite to answer the outstanding question of what shape ecological patterns in multi-species communities such as species-area relationships, relative species abundances, or spatial and temporal turnover of community composition; amid others [3]. To explain ecological patterns in nature, some rely on the concept that every species - through evolutionary processes and the acquisition of a unique set of traits that allow a species to be adapted to its abiotic and biotic environment - occupies a unique niche: Species coexistence comes as the result of niche differentiation [4,5]. Such a view has been challenged by the recognition of the key role of neutral processes [6], however, in which demographic stochasticity contributes to shape multi-species communities and to explain why congener species coexist much more frequently than expected by chance [7,8]. While the niche-based and neutral theories appear seemingly opposed at first sight [9], the dichotomy may be more philosophical than empirical [4,5]. Many examples have come to support that both concepts are not incompatible as they together influence the structure, diversity and functioning of communities [10], and are simply extreme cases of a continuum [11]. From this perspective, extrinsic factors, i.e., environmental heterogeneity, may influence the location of a given community along the niche-neutrality continuum. The walk of species in nature is therefore neither random nor ecologically predestined. In microbial assemblages, the co-existence of these two antagonistic mechanisms has been shown both theoretically and empirically. It has been shown that a combination of stabilising (niche) and equalising (neutral) mechanisms was responsible for the existence of groups of coexistent species (clumps) in a phytoplankton rich community [12]. Analysing interannual changes (2003-2009) in the weekly abundance of diatoms and dinoflagellates located in a temperate coastal ecosystem of the Western English Channel, Mutshinda et al. [13] found a mixture of biomass dynamics consistent with the neutrality-niche continuum hypothesis. While niche processes explained the dynamic of phytoplankton functional groups (i.e., diatoms vs. dinoflagellates) in terms of biomass, neutral processes mainly dominated - 50 to 75% of the time - the dynamics at the species level within functional groups [13]. From one endpoint to another, defining the location of a community along the continuum is all matter of scale [4,11]. In their study, testing predictions made by an emergent neutrality model, Graco-Roza et al. [14] provide empirical evidence that neutral and niche processes joined together to shape and drive planktonic communities in a riverine ecosystem. Body size - the 'master trait' - is used here as a discriminant ecological dimension along the niche axis. From their analysis, they not only show that the specific abundance is organised in clumps and gaps along the niche axis, but also reveal that different clumps exist along the river course. They identify two main clumps in body size - with species belonging to three different morphologically-based functional groups - and characterise that among-species differences in biovolume are driven by functional redundancy at the clump level; species functional distinctiveness being related to the relative biovolume of species. By grouping their variables according to seasons (cold-dry vs. warm-wet) or river elevation profile (upper, medium and lower course), they hereby highlight how environmental heterogeneity contributes to shape species assemblages and their dynamics and conclude that emergent neutrality models are a powerful approach to explain species coexistence; and therefore ecological patterns. References [1] Tea-makorn PP, Kosinski M (2020) Spouses’ faces are similar but do not become more similar with time. Scientific Reports, 10, 17001. https://doi.org/10.1038/s41598-020-73971-8. [2] MacArthur RH (1984) Geographical Ecology: Patterns in the Distribution of Species. Princeton University Press. [3] Vellend M (2020) The Theory of Ecological Communities (MPB-57). Princeton University Press. [4] Wennekes PL, Rosindell J, Etienne RS (2012) The Neutral—Niche Debate: A Philosophical Perspective. Acta Biotheoretica, 60, 257–271. https://doi.org/10.1007/s10441-012-9144-6. [5] Gravel D, Guichard F, Hochberg ME (2011) Species coexistence in a variable world. Ecology Letters, 14, 828–839. https://doi.org/10.1111/j.1461-0248.2011.01643.x. [6] Hubbell SP (2001) The Unified Neutral Theory of Biodiversity and Biogeography (MPB-32). Princeton University Press. [7] Leibold MA, McPeek MA (2006) Coexistence of the Niche and Neutral Perspectives in Community Ecology. Ecology, 87, 1399–1410. https://doi.org/10.1890/0012-9658(2006)87[1399:COTNAN]2.0.CO;2. [8] Pielou EC (1977) The Latitudinal Spans of Seaweed Species and Their Patterns of Overlap. Journal of Biogeography, 4, 299–311. https://doi.org/10.2307/3038189. [9] Holt RD (2006) Emergent neutrality. Trends in Ecology & Evolution, 21, 531–533. https://doi.org/10.1016/j.tree.2006.08.003. [10] Scheffer M, Nes EH van (2006) Self-organized similarity, the evolutionary emergence of groups of similar species. Proceedings of the National Academy of Sciences, 103, 6230–6235. https://doi.org/10.1073/pnas.0508024103. [11] Gravel D, Canham CD, Beaudet M, Messier C (2006) Reconciling niche and neutrality: the continuum hypothesis. Ecology Letters, 9, 399–409. https://doi.org/10.1111/j.1461-0248.2006.00884.x. [12] Vergnon R, Dulvy NK, Freckleton RP (2009) Niches versus neutrality: uncovering the drivers of diversity in a species-rich community. Ecology Letters, 12, 1079–1090. https://doi.org/10.1111/j.1461-0248.2009.01364.x. [13] Mutshinda CM, Finkel ZV, Widdicombe CE, Irwin AJ (2016) Ecological equivalence of species within phytoplankton functional groups. Functional Ecology, 30, 1714–1722. https://doi.org/10.1111/1365-2435.12641. [14] Graco-Roza C, Segura AM, Kruk C, Domingos P, Soininen J, Marinho MM (2021) Clumpy coexistence in phytoplankton: The role of functional similarity in community assembly. bioRxiv, 869966, ver. 6 peer-reviewed and recommended by Peer Community in Ecology. https://doi.org/10.1101/869966
| Clumpy coexistence in phytoplankton: The role of functional similarity in community assembly | Caio Graco-Roza, Angel M. Segura, Carla Kruk, Patricia Domingos, Janne Soininen, Marcelo M. Marinho | <p style="text-align: justify;">Emergent neutrality (EN) suggests that species must be sufficiently similar or sufficiently different in their niches to avoid interspecific competition. Such a scenario results in a transient pattern with clumps an... |  | Coexistence, Community ecology, Theoretical ecology | Cédric Hubas | 2020-01-23 16:11:32 | View | |
10 Aug 2023
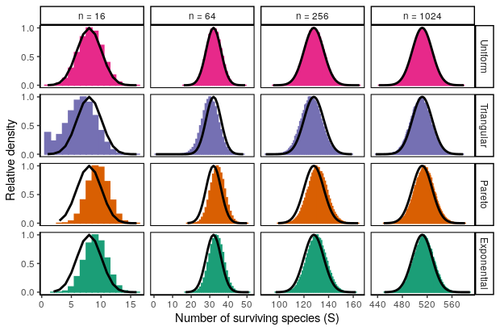
Coexistence of many species under a random competition-colonization trade-offZachary R. Miller, Maxime Clenet, Katja Della Libera, François Massol, Stefano Allesina https://doi.org/10.1101/2023.03.23.533867Assembly in metacommunities driven by a competition-colonization tradeoff: more species in, more species outRecommended by Frederik De Laender based on reviews by Canan Karakoç and 1 anonymous reviewerThe output of a community model depends on how you set its parameters. Thus, analyses of specific parameter settings hardwire the results to specific ecological scenarios. Because more general answers are often of interest, one tradition is to give models a statistical treatment: one summarizes how model parameters vary across species, and then predicts how changing the summary, instead of the individual parameters themselves, would change model output. Arguably the best-known example is the work initiated by May, showing that the properties of a community matrix, encoding effects species have on each other near their equilibrium, determine stability (1,2). More recently, this statistical treatment has also been applied to one of community ecology’s more prickly and slippery subjects: community assembly, which deals with the question “Given some regional species pool, which species will be able to persist together at some local ecosystem?”. Summaries of how species grow and interact in this regional pool predict the fraction of survivors and their relative abundances, the kind of dynamics, and various kinds of stability (3,4). One common characteristic of such statistical treatments is the assumption of disorder: if species do not interact in too structured ways, simple and therefore powerful predictions ensue that often stand up to scrutiny in relatively ordered systems. 2. Allesina, S. & Tang, S. (2015). The stability–complexity relationship at age 40: a random matrix perspective. Population Ecology, 57, 63–75. https://doi.org/10.1007/s10144-014-0471-0 3. Bunin, G. (2016). Interaction patterns and diversity in assembled ecological communities. Preprint at http://arxiv.org/abs/1607.04734. 5. Miller, Z. R., Clenet, M., Libera, K. D., Massol, F. & Allesina, S. (2023). Coexistence of many species under a random competition-colonization trade-off. bioRxiv 2023.03.23.533867, ver 3 peer-reviewed and recommended by PCI Ecology. https://doi.org/10.1101/2023.03.23.533867 6. Serván, C. A. & Allesina, S. (2021). Tractable models of ecological assembly. Ecology Letters, 24, 1029–1037. https://doi.org/10.1111/ele.13702 | Coexistence of many species under a random competition-colonization trade-off | Zachary R. Miller, Maxime Clenet, Katja Della Libera, François Massol, Stefano Allesina | <p>The competition-colonization trade-off is a well-studied coexistence mechanism for metacommunities. In this setting, it is believed that coexistence of all species requires their traits to satisfy restrictive conditions limiting their similarit... |  | Biodiversity, Coexistence, Colonization, Community ecology, Competition, Population ecology, Spatial ecology, Metacommunities & Metapopulations, Theoretical ecology | Frederik De Laender | 2023-03-30 20:42:48 | View | |
16 Jun 2023
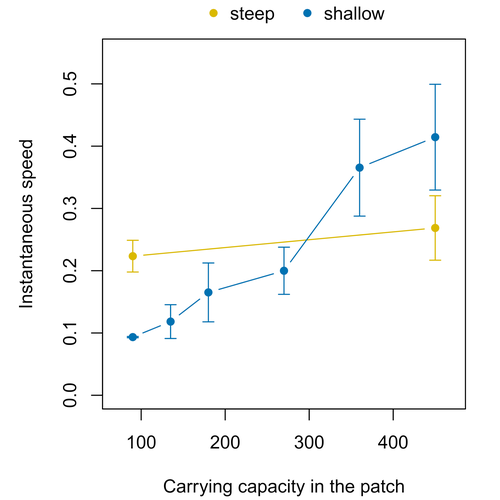
Colonisation debt: when invasion history impacts current range expansionThibaut Morel-Journel, Marjorie Haond, Lana Duan, Ludovic Mailleret, Elodie Vercken https://doi.org/10.1101/2022.11.13.516255Combining stochastic models and experiments to understand dispersal in heterogeneous environmentsRecommended by Joaquín Hortal based on reviews by 2 anonymous reviewersDispersal is a key element of the natural dynamics of meta-communities, and plays a central role in the success of populations colonizing new landscapes. Understanding how demographic processes may affect the speed at which alien species spread through environmentally-heterogeneous habitat fragments is therefore of key importance to manage biological invasions. This requires studying together the complex interplay of dispersal and population processes, two inextricably related phenomena that can produce many possible outcomes. Stochastic models offer an opportunity to describe this kind of process in a meaningful way, but to ensure that they are realistic (sensu Levins 1966) it is also necessary to combine model simulations with empirical data (Snäll et al. 2007). Morel-Journel et al. (2023) put together stochastic models and experimental data to study how population density may affect the speed at which alien species spread through a heterogeneous landscape. They do it by focusing on what they call ‘colonisation debt’, which is merely the impact that population density at the invasion front may have on the speed at which the species colonizes patches of different carrying capacities. They investigate this issue through two largely independent approaches. First, a stochastic model of dispersal throughout the patches of a linear, 1-dimensional landscape, which accounts for different degrees of density-dependent growth. And second, a microcosm experiment of a parasitoid wasp colonizing patches with different numbers of host eggs. In both cases, they compare the velocity of colonization of patches with lower or higher carrying capacity than the previous one (i.e. what they call upward or downward gradients). Their results show that density-dependent processes influence the speed at which new fragments are colonized is significantly reduced by positive density dependence. When either population growth or dispersal rate depend on density, colonisation debt limits the speed of invasion, which turns out to be dependent on the strength and direction of the gradient between the conditions of the invasion front, and the newly colonized patches. Although this result may be quite important to understand the meta-population dynamics of dispersing species, it is important to note that in their study the environmental differences between patches do not take into account eventual shifts in the scenopoetic conditions (i.e. the values of the environmental parameters to which species niches’ respond to; Hutchinson 1978, see also Soberón 2007). Rather, differences arise from variations in the carrying capacity of the patches that are consecutively invaded, both in the in silico and microcosm experiments. That is, they account for potential differences in the size or quality of the invaded fragments, but not on the costs of colonizing fragments with different environmental conditions, which may also determine invasion speed through niche-driven processes. This aspect can be of particular importance in biological invasions or under climate change-driven range shifts, when adaptation to new environments is often required (Sakai et al. 2001; Whitney & Gabler 2008; Hill et al. 2011). The expansion of geographical distribution ranges is the result of complex eco-evolutionary processes where meta-community dynamics and niche shifts interact in a novel physical space and/or environment (see, e.g., Mestre et al. 2020). Here, the invasibility of native communities is determined by niche variations and how similar are the traits of alien and native species (Hui et al. 2023). Within this context, density-dependent processes will build upon and heterogeneous matrix of native communities and environments (Tischendorf et al. 2005), to eventually determine invasion success. What the results of Morel-Journel et al. (2023) show is that, when the invader shows density dependence, the invasion process can be slowed down by variations in the carrying capacity of patches along the dispersal front. This can be particularly useful to manage biological invasions; ongoing invasions can be at least partially controlled by manipulating the size or quality of the patches that are most adequate to the invader, controlling host populations to reduce carrying capacity. But further, landscape manipulation of such kind could be used in a preventive way, to account in advance for the effects of the introduction of alien species for agricultural exploitation or biological control, thereby providing an additional safeguard to practices such as the introduction of parasitoids to control plagues. These practical aspects are certainly worth exploring further, together with a more explicit account of the influence of the abiotic conditions and the characteristics of the invaded communities on the success and speed of biological invasions. REFERENCES Hill, J.K., Griffiths, H.M. & Thomas, C.D. (2011) Climate change and evolutionary adaptations at species' range margins. Annual Review of Entomology, 56, 143-159. https://doi.org/10.1146/annurev-ento-120709-144746 Hui, C., Pyšek, P. & Richardson, D.M. (2023) Disentangling the relationships among abundance, invasiveness and invasibility in trait space. npj Biodiversity, 2, 13. https://doi.org/10.1038/s44185-023-00019-1 Hutchinson, G.E. (1978) An introduction to population biology. Yale University Press, New Haven, CT. Levins, R. (1966) The strategy of model building in population biology. American Scientist, 54, 421-431. Mestre, A., Poulin, R. & Hortal, J. (2020) A niche perspective on the range expansion of symbionts. Biological Reviews, 95, 491-516. https://doi.org/10.1111/brv.12574 Morel-Journel, T., Haond, M., Duan, L., Mailleret, L. & Vercken, E. (2023) Colonisation debt: when invasion history impacts current range expansion. bioRxiv, 2022.11.13.516255, ver. 3 peer-reviewed and recommended by Peer Community in Ecology. https://doi.org/10.1101/2022.11.13.516255 Snäll, T., B. O'Hara, R. & Arjas, E. (2007) A mathematical and statistical framework for modelling dispersal. Oikos, 116, 1037-1050. https://doi.org/10.1111/j.0030-1299.2007.15604.x Sakai, A.K., Allendorf, F.W., Holt, J.S., Lodge, D.M., Molofsky, J., With, K.A., Baughman, S., Cabin, R.J., Cohen, J.E., Ellstrand, N.C., McCauley, D.E., O'Neil, P., Parker, I.M., Thompson, J.N. & Weller, S.G. (2001) The population biology of invasive species. Annual Review of Ecology and Systematics, 32, 305-332. https://doi.org/10.1146/annurev.ecolsys.32.081501.114037 Soberón, J. (2007) Grinnellian and Eltonian niches and geographic distributions of species. Ecology Letters, 10, 1115-1123. https://doi.org/10.1111/j.1461-0248.2007.01107.x Tischendorf, L., Grez, A., Zaviezo, T. & Fahrig, L. (2005) Mechanisms affecting population density in fragmented habitat. Ecology and Society, 10, 7. https://doi.org/10.5751/ES-01265-100107 Whitney, K.D. & Gabler, C.A. (2008) Rapid evolution in introduced species, 'invasive traits' and recipient communities: challenges for predicting invasive potential. Diversity and Distributions, 14, 569-580. https://doi.org/10.1111/j.1472-4642.2008.00473.x | Colonisation debt: when invasion history impacts current range expansion | Thibaut Morel-Journel, Marjorie Haond, Lana Duan, Ludovic Mailleret, Elodie Vercken | <p>Demographic processes that occur at the local level, such as positive density dependence in growth or dispersal, are known to shape population range expansion, notably by linking carrying capacity to invasion speed. As a result of these process... |  | Biological invasions, Colonization, Dispersal & Migration, Experimental ecology, Landscape ecology, Population ecology, Spatial ecology, Metacommunities & Metapopulations, Theoretical ecology | Joaquín Hortal | Anonymous, Anonymous | 2022-11-16 15:52:08 | View |
11 Mar 2022
Comment on “Information arms race explains plant-herbivore chemical communication in ecological communities”Ethan Bass, André Kessler https://doi.org/10.32942/osf.io/xsbtmDoes information theory inform chemical arms race communication?Recommended by Rodrigo Medel based on reviews by Claudio Ramirez and 2 anonymous reviewersOne of the long-standing questions in evolutionary ecology is on the mechanisms involved in arms race coevolution. One way to address this question is to understand the conditions under which one species evolves traits in response to the presence of a second species and so on. However, specialized pairwise interactions are by far less common in nature than interactions involving a higher number of interacting species (Bascompte, Jordano 2013). While interactions between large sets of species are the norm rather than the exception in mutualistic (pollination, seed dispersal), and antagonist (herbivory, parasitism) relationships, few is known on the way species identify, process, and respond to information provided by other interacting species under field conditions (Schaefer, Ruxton 2011). Zu et al. (2020) addressed this general question by developing an interesting information theory-based approach that hypothesized conditional entropy in chemical communication plays a role as proxy of fitness in plant-herbivore communities. More specifically, plant fitness was assumed to be related to the efficiency to code signals by plant species, and herbivore fitness to the capacity to decode plant signals. In this way, from the plant perspective, the elaboration of plant signals that elude decoding by herbivores is expected to be favored, as herbivores are expected to attack plants with simple chemical signals. The empirical observation upon which the model was tested was the redundancy in volatile organic compounds (VOC) found across plant species in a plant-herbivore community. Interestingly, Zu et al.’s model predicted successfully that VOC redundancy in the plant community associates with increased conditional entropy, which conveys herbivore confusion and plant protection against herbivory. In this way, plant species that evolve VOCs already present in the community might be benefitted, ultimately leading to the patterns of VOC redundancy commonly observed in nature. Bass & Kessler performed a series of interesting observations on Zu et al. (2020), that can be organized along three lines of reasoning. First, from an evolutionary perspective, Bass & Kessler note the important point that accepting that conditional information entropy, estimated from the contribution of every plant species to volatile redundancy implies that average plant fitness seems to depend on community-level properties (i.e., what the other species in the community are doing) rather than on population-level characteristics (I.e., what the individuals belonging a population are doing). While the level at which selection acts upon is a longstanding debate (e.g., Goodnight, 1990; Williams, 1992), the model seems to contradict one of the basic tenets of Darwinian evolution. The extent to which this important observation invalidates the contribution of Zu et al. (2020) is open to scrutiny. However, one can indulge the evolutionary criticism by arguing that every theoretical model performs a number of assumptions to preserve the simplicity of analyses. Furthermore, even accepting the criticism, the overall information-based framework is valuable as it provides a fresh perspective to the way coding and decoding chemical information in plant-herbivore interactions may result in arm race coevolution. The question to be assessed by members of the scientific community is how strong the evolutionary assumptions are to be acceptable. A second line of reasoning involves consideration of additional routes of chemical information transfer. If chemical volatiles are involved in another ecological function unrelated to arm race (as they are) such as toxicity, crypsis, aposematism, etc., the conditional information indices considered as proxy to plant and herbivore fitness may be only secondarily related to arms race. This is an interesting observation, which suggests that VOC production may have more than one ecological function, as it often happens in “pleiotropic” traits (Strauss, Irwin 2004). This is an exciting avenue for future research. Finally, a third category of comments involves the relationship between conditional information entropy and plant and herbivore fitness. Bass & Kessler developed a Bayesian treatment of the community-level information developed by Zu et al. (2020) that permitted to estimate fitness on a species rather than community level. Their results revealed that community conditional entropies fail to align with species-level indices, suggesting that conclusions of Strauss & Irwin (2004) are not commensurate with fitness at the species level, where the analysis seems to be pertinent. In general, I strongly recommend Bass & Kessler’s contribution as it provides a series of observations and new perspectives to Zu et al. (2020). Rather than restricting their manuscript to blind criticisms, Bass & Kessler provides new interesting perspectives, which is always welcome as it improves the value and scope of the original work. References Bascompte J, Jordano P (2013) Mutualistic Networks. Princeton University Press. https://doi.org/10.23943/princeton/9780691131269.001.0001 Bass E, Kessler A (2022) Comment on “Information arms race explains plant-herbivore chemical communication in ecological communities.” EcoEvoRxiv, ver. 8 peer-reviewed and recommended by Peer Community in Ecology. https://doi.org/10.32942/osf.io/xsbtm Goodnight CJ (1990) Experimental Studies of Community Evolution I: The Response to Selection at the Community Level. Evolution, 44, 1614–1624. https://doi.org/10.1111/j.1558-5646.1990.tb03850.x Schaefer HM, Ruxton GD (2011) Plant-Animal Communication. Oxford University Press, Oxford. https://doi.org/10.1093/acprof:osobl/9780199563609.001.0001 Strauss SY, Irwin RE (2004) Ecological and Evolutionary Consequences of Multispecies Plant-Animal Interactions. Annual Review of Ecology, Evolution, and Systematics, 35, 435–466. https://doi.org/10.1146/annurev.ecolsys.35.112202.130215 Williams GC (1992) Natural Selection: Domains, Levels, and Challenges. Oxford University Press, Oxford, New York. Zu P, Boege K, del-Val E, Schuman MC, Stevenson PC, Zaldivar-Riverón A, Saavedra S (2020) Information arms race explains plant-herbivore chemical communication in ecological communities. Science, 368, 1377–1381. https://doi.org/10.1126/science.aba2965 | Comment on “Information arms race explains plant-herbivore chemical communication in ecological communities” | Ethan Bass, André Kessler | <p style="text-align: justify;">Zu et al (Science, 19 Jun 2020, p. 1377) propose that an ‘information arms-race’ between plants and herbivores explains plant-herbivore communication at the community level. However, the analysis presented here show... |  | Chemical ecology, Community ecology, Eco-evolutionary dynamics, Evolutionary ecology, Herbivory, Interaction networks, Theoretical ecology | Rodrigo Medel | 2021-10-02 06:06:07 | View | |
27 May 2019
Community size affects the signals of ecological drift and selection on biodiversityTadeu Siqueira, Victor S. Saito, Luis M. Bini, Adriano S. Melo, Danielle K. Petsch, Victor L. Landeiro, Kimmo T. Tolonen, Jenny Jyrkänkallio-Mikkola, Janne Soininen, Jani Heino https://doi.org/10.1101/515098Toward an empirical synthesis on the niche versus stochastic debateRecommended by Eric Harvey based on reviews by Kevin Cazelles and Romain BertrandAs far back as Clements [1] and Gleason [2], the historical schism between deterministic and stochastic perspectives has divided ecologists. Deterministic theories tend to emphasize niche-based processes such as environmental filtering and species interactions as the main drivers of species distribution in nature, while stochastic theories mainly focus on chance colonization, random extinctions and ecological drift [3]. Although the old days when ecologists were fighting fiercely over null models and their adequacy to capture niche-based processes is over [4], the ghost of that debate between deterministic and stochastic perspectives came back to haunt ecologists in the form of the ‘environment versus space’ debate with the development of metacommunity theory [5]. While interest in that question led to meaningful syntheses of metacommunity dynamics in natural systems [6], it also illustrated how context-dependant the answer was [7]. One of the next frontiers in metacommunity ecology is to identify the underlying drivers of this observed context-dependency in the relative importance of ecological processus [7, 8]. References [1] Clements, F. E. (1936). Nature and structure of the climax. Journal of ecology, 24(1), 252-284. doi: 10.2307/2256278 | Community size affects the signals of ecological drift and selection on biodiversity | Tadeu Siqueira, Victor S. Saito, Luis M. Bini, Adriano S. Melo, Danielle K. Petsch, Victor L. Landeiro, Kimmo T. Tolonen, Jenny Jyrkänkallio-Mikkola, Janne Soininen, Jani Heino | <p>Ecological drift can override the effects of deterministic niche selection on small populations and drive the assembly of small communities. We tested the hypothesis that smaller local communities are more dissimilar among each other because of... | Biodiversity, Coexistence, Community ecology, Competition, Conservation biology, Dispersal & Migration, Freshwater ecology, Spatial ecology, Metacommunities & Metapopulations | Eric Harvey | 2019-01-09 19:06:21 | View | ||
06 Jan 2021

Comparing statistical and mechanistic models to identify the drivers of mortality within a rear-edge beech populationCathleen Petit-Cailleux, Hendrik Davi, François Lefevre, Christophe Hurson, Joseph Garrigue, Jean-André Magdalou, Elodie Magnanou and Sylvie Oddou-Muratorio https://doi.org/10.1101/645747The complexity of predicting mortality in treesRecommended by Lucía DeSoto based on reviews by Lisa Hülsmann and 2 anonymous reviewersOne of the main issues of forest ecosystems is rising tree mortality as a result of extreme weather events (Franklin et al., 1987). Eventually, tree mortality reduces forest biomass (Allen et al., 2010), although its effect on forest ecosystem fluxes seems not lasting too long (Anderegg et al., 2016). This controversy about the negative consequences of tree mortality is joined to the debate about the drivers triggering and the mechanisms accelerating tree decline. For instance, there is still room for discussion about carbon starvation or hydraulic failure determining the decay processes (Sevanto et al., 2014) or about the importance of mortality sources (Reichstein et al., 2013). Therefore, understanding and predicting tree mortality has become one of the challenges for forest ecologists in the last decade, doubling the rate of articles published on the topic (*). Although predicting the responses of ecosystems to environmental change based on the traits of species may seem a simplistic conception of ecosystem functioning (Sutherland et al., 2013), identifying those traits that are involved in the proneness of a tree to die would help to predict how forests will respond to climate threatens. (*) Number (and percentage) of articles found in Web of Sciences after searching (December the 10th, 2020) “tree mortality”: from 163 (0.006%) in 2010 to 412 (0.013%) in 2020. References Allen et al. (2010). A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. Forest ecology and management, 259(4), 660-684. doi: https://doi.org/10.1016/j.foreco.2009.09.001 | Comparing statistical and mechanistic models to identify the drivers of mortality within a rear-edge beech population | Cathleen Petit-Cailleux, Hendrik Davi, François Lefevre, Christophe Hurson, Joseph Garrigue, Jean-André Magdalou, Elodie Magnanou and Sylvie Oddou-Muratorio | <p>Since several studies have been reporting an increase in the decline of forests, a major issue in ecology is to better understand and predict tree mortality. The interactions between the different factors and the physiological processes giving ... |  | Climate change, Physiology, Population ecology | Lucía DeSoto | 2019-05-24 11:37:38 | View | |
31 May 2023
Conservation networks do not match the ecological requirements of amphibiansMatutini Florence, Jacques Baudry, Marie-Josée Fortin, Guillaume Pain, Joséphine Pithon https://doi.org/10.1101/2022.07.18.500425Amphibians under scrutiny - When human-dominated landscape mosaics are not in full compliance with their ecological requirementsRecommended by Sandrine Charles based on reviews by Peter Vermeiren and 1 anonymous reviewer based on reviews by Peter Vermeiren and 1 anonymous reviewer
Among vertebrates, amphibians are one of the most diverse groups with more than 7,000 known species. Amphibians occupy various ecosystems, including forests, wetlands, and freshwater habitats. Amphibians are known to be highly sensitive to changes in their environment, particularly to water quality and habitat degradation, so that monitoring abundance of amphibian populations can provide early warning signs of ecosystem disturbances that may also affect other organisms including humans (Bishop et al., 2012). Accordingly, efforts in habitat preservation and sustainable land and water management are necessary to safeguard amphibian populations. In this context, Matutini et al. (2023) compared ecological requirements of amphibian species with the quality of agricultural landscape mosaics. Doing so, they identified critical gaps in existing conservation tools that include protected areas, green infrastructures, and inventoried sites. Matutini et al. (2023) focused on nine amphibian species in the Pays-de-la-Loire region where the landscape has been fashioned over the years by human activities. Three of the chosen amphibian species are living in a dense hedgerow mosaic landscape, while five others are more generalists. Matutini et al. (2023) established multi-species habitat suitability maps, together with their levels of confidence, by combining single species maps with a probabilistic stacking method at 500-m resolution. From these maps, habitats were classified in five categories, from not suitable to highly suitable. Then, the circuit theory was used to map the potential connections between each highly suitable patch at the regional scale. Finally, comparing suitability maps with existing conservation tools, Matutini et al. (2023) were able to assess their coverage and efficiency. Whatever their species status (endangered or not), Matutini et al. (2023) highlighted some discrepancies between the ecological requirements of amphibians in terms of habitat quality and the conservation tools of the landscape mosaic within which they are evolving. More specifically, Matutini et al. (2023) found that protected areas and inventoried sites covered only a small proportion of highly suitable habitats, while green infrastructures covered around 50% of the potential habitat for amphibian species. Such a lack of coverage and efficiency of protected areas brings to light that geographical sites with amphibian conservation challenges are known but not protected. Regarding the landscape fragmentation, Matutini et al. (2023) found that generalist amphibian species have a more homogeneous distribution of suitable habitats at the regional scale. They also identified two bottlenecks between two areas of suitable habitats, a situation that could prove critical to amphibian movements if amphibians were forced to change habitats to global change. In conclusion, Matutini et al. (2023) bring convincing arguments in support of land-use species-conservation planning based on a better consideration of human-dominated landscape mosaics in full compliance with ecological requirements of the species that inhabit the regions concerned. ReferencesBishop, P.J., Angulo, A., Lewis, J.P., Moore, R.D., Rabb, G.B., Moreno, G., 2012. The Amphibian Extinction Crisis - what will it take to put the action into the Amphibian Conservation Action Plan? Sapiens - Surveys and Perspectives Integrating Environment and Society 5, 1–16. http://journals.openedition.org/sapiens/1406 Matutini, F., Baudry, J., Fortin, M.-J., Pain, G., Pithon, J., 2023. Conservation networks do not match ecological requirements of amphibians. bioRxiv, ver. 3 peer-reviewed and recommended by Peer Community in Ecology. https://doi.org/10.1101/2022.07.18.500425 | Conservation networks do not match the ecological requirements of amphibians | Matutini Florence, Jacques Baudry, Marie-Josée Fortin, Guillaume Pain, Joséphine Pithon | <p style="text-align: justify;">1. Amphibians are among the most threatened taxa as they are highly sensitive to habitat degradation and fragmentation. They are considered as model species to evaluate habitats quality in agricultural landscapes. I... | Biodiversity, Biogeography, Human impact, Landscape ecology, Macroecology, Spatial ecology, Metacommunities & Metapopulations, Species distributions, Terrestrial ecology | Sandrine Charles | 2022-09-20 14:40:03 | View | ||
24 Nov 2023
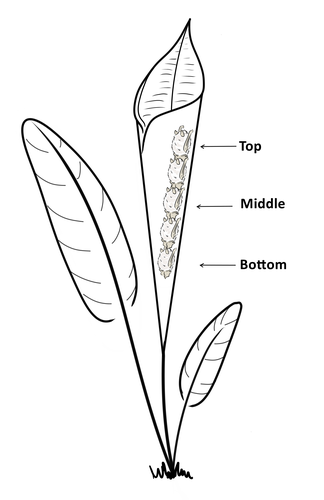
Consistent individual positions within roosts in Spix's disc-winged batsGiada Giacomini, Silvia Chaves-Ramirez, Andres Hernandez-Pinson, Jose Pablo Barrantes, Gloriana Chaverri https://doi.org/10.1101/2022.11.04.515223Consistent individual differences in habitat use in a tropical leaf roosting batRecommended by Corina Logan based on reviews by Annemarie van der Marel and 2 anonymous reviewersConsistent individual differences in habitat use are found across species and can play a role in who an individual mates with, their risk of predation, and their ability to compete with others (Stuber et al. 2022). However, the data informing such hypotheses come primarily from temperate regions (Stroud & Thompson 2019, Titley et al. 2017). This calls into question the generalizability of the conclusions from this research until further investigations can be conducted in tropical regions. Giacomini and colleagues (2023) tackled this task in an investigation of consistent individual differences in habitat use in the Central American tropics. They explored whether Spix’s disc-winged bats form positional hierarchies in roosts, which is an excellent start to learning more about the social behavior of this species - a species that is difficult to directly observe. They found that individual bats use their roosting habitat in predictable ways by positioning themselves consistently either in the bottom, middle, or top of the roost leaf. Individuals chose the same positions across time and across different roost sites. They also found that age and sex play a role in which sections individuals are positioned in. Their research shows that consistent individual differences in habitat use are present in a tropical system, and sets the stage for further investigations into social behavior in this species, particularly whether there is a dominance hierarchy among individuals and whether some positions in the roost are more protective and sought after than others. References Giacomini G, Chaves-Ramirez S, Hernandez-Pinson A, Barrantes JP, Chaverri G. (2023). Consistent individual positions within roosts in Spix's disc-winged bats. bioRxiv, https://doi.org/10.1101/2022.11.04.515223 Stroud, J. T., & Thompson, M. E. (2019). Looking to the past to understand the future of tropical conservation: The importance of collecting basic data. Biotropica, 51(3), 293-299. https://doi.org/10.1111/btp.12665 Stuber, E. F., Carlson, B. S., & Jesmer, B. R. (2022). Spatial personalities: a meta-analysis of consistent individual differences in spatial behavior. Behavioral Ecology, 33(3), 477-486. https://doi.org/10.1093/beheco/arab147 Titley, M. A., Snaddon, J. L., & Turner, E. C. (2017). Scientific research on animal biodiversity is systematically biased towards vertebrates and temperate regions. PloS one, 12(12), e0189577. https://doi.org/10.1371/journal.pone.0189577 | Consistent individual positions within roosts in Spix's disc-winged bats | Giada Giacomini, Silvia Chaves-Ramirez, Andres Hernandez-Pinson, Jose Pablo Barrantes, Gloriana Chaverri | <p style="text-align: justify;">Individuals within both moving and stationary groups arrange themselves in a predictable manner; for example, some individuals are consistently found at the front of the group or in the periphery and others in the c... |  | Behaviour & Ethology, Social structure, Zoology | Corina Logan | 2022-11-05 17:39:35 | View | |
14 Jan 2021
Consistent variations in personality traits and their potential for genetic improvement of biocontrol agents: Trichogramma evanescens as a case studySilène Lartigue, Myriam Yalaoui, Jean Belliard, Claire Caravel, Louise Jeandroz, Géraldine Groussier, Vincent Calcagno, Philippe Louâpre, François-Xavier Dechaume-Moncharmont, Thibaut Malausa and Jérôme Moreau https://doi.org/10.1101/2020.08.21.257881Tell us how you can be, and we’ll make you better: exploiting genetic variability in personality traits to improve top-down control of agricultural pestsRecommended by Marta Montserrat based on reviews by Bart A Pannebakker, François Dumont, Joshua Patrick Byrne and Ana Pimenta Goncalves PereiraAgriculture in the XXI century faces the huge challenge of having to provide food to a rapidly growing human population, which is expected to reach 10.9 billion in 2100 (UUNN 2019), by means of practices and methods that guarantee crop sustainability, human health safety, and respect to the environment (UUNN 2015). Such regulation by the United Nations ultimately entails that agricultural scientists are urged to design strategies and methods that effectively minimize the use of harmful chemical products to control pest populations and to improve soil quality. References Bielza, P., Balanza, V., Cifuentes, D. and Mendoza, J. E. (2020). Challenges facing arthropod biological control: Identifying traits for genetic improvement of predators in protected crops. Pest Manag Sci. doi: https://doi.org/10.1002/ps.5857 | Consistent variations in personality traits and their potential for genetic improvement of biocontrol agents: Trichogramma evanescens as a case study | Silène Lartigue, Myriam Yalaoui, Jean Belliard, Claire Caravel, Louise Jeandroz, Géraldine Groussier, Vincent Calcagno, Philippe Louâpre, François-Xavier Dechaume-Moncharmont, Thibaut Malausa and Jérôme Moreau | <p>Improvements in the biological control of agricultural pests require improvements in the phenotyping methods used by practitioners to select efficient biological control agent (BCA) populations in industrial rearing or field conditions. Consist... | Agroecology, Behaviour & Ethology, Biological control, Evolutionary ecology, Life history | Marta Montserrat | 2020-08-24 10:40:03 | View | ||
24 May 2022
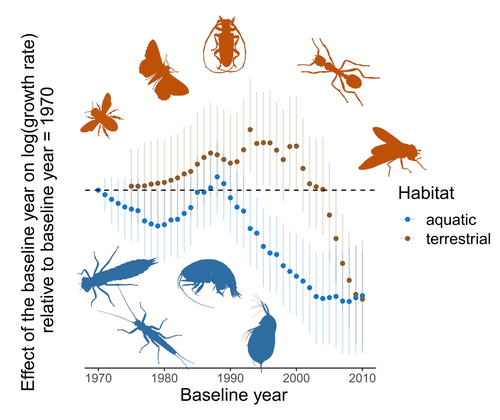
Controversy over the decline of arthropods: a matter of temporal baseline?François Duchenne, Emmanuelle Porcher, Jean-Baptiste Mihoub, Grégoire Loïs, Colin Fontaine https://doi.org/10.1101/2022.02.09.479422Don't jump to conclusions on arthropod abundance dynamics without appropriate dataRecommended by Tim Coulson based on reviews by Gabor L Lovei and 1 anonymous reviewer based on reviews by Gabor L Lovei and 1 anonymous reviewer
Humans are dramatically modifying many aspects of our planet via increasing concentrations of carbon dioxide in the atmosphere, patterns of land-use change, and unsustainable exploitation of the planet’s resources. These changes impact the abundance of species of wild organisms, with winners and losers. Identifying how different species and groups of species are influenced by anthropogenic activity in different biomes, continents, and habitats, has become a pressing scientific question with many publications reporting analyses of disparate data on species population sizes. Many conclusions are based on the linear analysis of rather short time series of organismal abundances. Duchenne F, Porcher E, Mihoub J-B, Loïs G, Fontaine C (2022) Controversy over the decline of arthropods: a matter of temporal baseline? bioRxiv, 2022.02.09.479422, ver. 3 peer-reviewed and recommended by Peer Community in Ecology. https://doi.org/10.1101/2022.02.09.479422 | Controversy over the decline of arthropods: a matter of temporal baseline? | François Duchenne, Emmanuelle Porcher, Jean-Baptiste Mihoub, Grégoire Loïs, Colin Fontaine | <p style="text-align: justify;">Recently, a number of studies have reported somewhat contradictory patterns of temporal trends in arthropod abundance, from decline to increase. Arthropods often exhibit non-monotonous variation in abundance over ti... |  | Conservation biology | Tim Coulson | 2022-02-11 15:44:44 | View |
MANAGING BOARD
Julia Astegiano
Tim Coulson
Anna Eklof
Dominique Gravel
François Massol
Ben Phillips
Cyrille Violle










