Detecting within-host interactions using genotype combination prevalence data
Samuel Alizon, Carmen Lía Murall, Emma Saulnier, Mircea T Sofonea
https://doi.org/10.1101/256586
Combining epidemiological models with statistical inference can detect parasite interactions
Recommended by Dustin Brisson based on reviews by Samuel Díaz Muñoz, Erick Gagne and 1 anonymous reviewer
There are several important topics in the study of infectious diseases that have not been well explored due to technical difficulties. One such topic is pursued by Alizon et al. in “Modelling coinfections to detect within-host interactions from genotype combination prevalences” [1]. Both theory and several important examples have demonstrated that interactions among co-infecting strains can have outsized impacts on disease outcomes, transmission dynamics, and epidemiology. Unfortunately, empirical data on pathogen interactions and their outcomes is often correlational making results difficult to decipher.
The analytical framework developed by Alizon et al. [1] infers the presence and strength of pathogen interactions through their impact on transmission dynamics using a novel application of Approximate Bayesian Computation (ABC)-regression to epidemiological data. Traditional analytic approaches identify pathogen interactions when the observed distribution of pathogens among hosts differ from ‘neutral’ expectations. However, deviations from this expectation are not only a result of inter-strain interactions but can be caused by many ecological interactions, such as heterogeneity in host contact networks. To overcome this difficulty, Alizon et al [1] develop an analytical framework that incorporates explicit epidemiological models to allow inference of interactions among strains of Human Papillomaviruses (HPV) even with other ecological interactions that impact the distribution of strains among hosts. Alizon et al also demonstrate that using more of the available data, including the specific combination of strains present in hosts and knowledge of the connectivity of the hosts (i.e., super-spreaders), leads to more accurate inferences of the strength and direction of within-host interactions among coinfecting strains. This method successfully identified data generated from models with high and moderate inter-strain interaction intensity when the host population was homogeneous and was only slightly less successful when the host population was heterogeneous (super-spreaders present). By comparison, some previously published analytical methods could identify only some inter-strain interactions in datasets generated from models with homogeneous host populations, but host heterogeneity obscured these interactions.
This manuscript makes seamless connections between basic viral biology and its epidemiological consequences by tying them together with realistic models, illustrating the fundamental utility of biological modeling. This analytical framework provides crucial tools for experimentalists, facilitating collaborations with theoreticians to better understand the epidemiological consequences of co-infections. In addition, the method is simple enough to be applied by a broad base of experimentalists to the many pathogens where co-infections are common. Thus, this paper has the potential to impact several research fields and public health practice. Those attempting to apply this method should note the potential limitations noted by the authors. For example, it is not designed to detect the mechanisms of inter-strain interactions (there is no within host component of the models) but to identify the existence of interactions through patterns indicative of these interactions while ruling out other sources that could cause the pattern. This approach is likely to be most accurate when strain identification within hosts is precise and unbiased - which is unlikely in many systems where samples are taken only from symptomatic cases and strain detection is not sufficiently sensitive – and when host contact networks can be reasonably estimated. Importantly, a priori knowledge of the set of possible epidemiological models is needed for accurate parameter estimates, which may be true for several prominent pathogens, but not be so for many other pathogens and symbionts. We look forward to future extensions of this framework where this restriction is relaxed. Alizon et al. [1] have provided a framework that will facilitate theoretical and empirical work on the impact of coinfections on infectious disease and should shape future public health data collection standards.
References
[1] Alizon, S., Murall, C.L., Saulnier, E., & Sofonea, M.T. (2018). Detecting within-host interactions using genotype combination prevalence data. bioRxiv, 256586, ver. 3 peer-reviewed and recommended by PCI Ecology. doi: 10.1101/256586
| Detecting within-host interactions using genotype combination prevalence data | Samuel Alizon, Carmen Lía Murall, Emma Saulnier, Mircea T Sofonea | <p>Parasite genetic diversity can provide information on disease transmission dynamics but most methods ignore the exact combinations of genotypes in infections. We introduce and validate a new method that combines explicit epidemiological modelli... |  | Eco-immunology & Immunity, Epidemiology, Host-parasite interactions, Statistical ecology | Dustin Brisson | Samuel Díaz Muñoz, Erick Gagne | 2018-02-01 09:23:26 | View |
Data-based, synthesis-driven: setting the agenda for computational ecology
Timothée Poisot, Richard Labrie, Erin Larson, Anastasia Rahlin
10.1101/150128
Some thoughts on computational ecology from people who I’m sure use different passwords for each of their accounts
Recommended by Phillip P.A. Staniczenko based on reviews by Matthieu Barbier and 1 anonymous reviewer
Are you an ecologist who uses a computer or know someone that does?
Even if your research doesn’t rely heavily on advanced computational techniques, it likely hasn’t escaped your attention that computers are increasingly being used to analyse field data and make predictions about the consequences of environmental change. So before artificial intelligence and robots take over from scientists, now is great time to read about how experts think computers could make your life easier and lead to innovations in ecological research.
In “Data-based, synthesis-driven: setting the agenda for computational ecology”, Poisot and colleagues [1] provide a brief history of computational ecology and offer their thoughts on how computational thinking can help to bridge different types of ecological knowledge.
In this wide-ranging article, the authors share practical strategies for realising three main goals: (i) tighter integration of data and models to make predictions that motivate action by practitioners and policy-makers; (ii) closer interaction between data-collectors and data-users; and (iii) enthusiasm and aptitude for computational techniques in future generations of ecologists.
The key, Poisot and colleagues argue, is for ecologists to “engage in meaningful dialogue across disciplines, and recognize the currencies of their collaborations.”
Yes, this is easier said than done. However, the journey is much easier with a guide and when everyone involved serves to benefit not only from the eventual outcome, but also the process.
References
[1] Poisot, T., Labrie, R., Larson, E., & Rahlin, A. (2018). Data-based, synthesis-driven: setting the agenda for computational ecology. BioRxiv, 150128, ver. 4 recommended and peer-reviewed by PCI Ecology. doi: 10.1101/150128
| Data-based, synthesis-driven: setting the agenda for computational ecology | Timothée Poisot, Richard Labrie, Erin Larson, Anastasia Rahlin | Computational ecology, defined as the application of computational thinking to ecological problems, has the potential to transform the way ecologists think about the integration of data and models. As the practice is gaining prominence as a way to... |  | Meta-analyses, Statistical ecology, Theoretical ecology | Phillip P.A. Staniczenko | | 2018-02-05 20:51:41 | View |
How optimal foragers should respond to habitat changes? On the consequences of habitat conversion.
Vincent Calcagno, Frederic Hamelin, Ludovic Mailleret, Frederic Grognard
10.1101/273557
Optimal foraging in a changing world: old questions, new perspectives
Recommended by Francois-Xavier Dechaume-Moncharmont based on reviews by Frederick Adler, Andrew Higginson and 1 anonymous reviewer based on reviews by Frederick Adler, Andrew Higginson and 1 anonymous reviewer
Marginal value theorem (MVT) is an archetypal model discussed in every behavioural ecology textbook. Its popularity is largely explained but the fact that it is possible to solve it graphically (at least in its simplest form) with the minimal amount of equations, which is a sensible strategy for an introductory course in behavioural ecology [1]. Apart from this heuristic value, one may be tempted to disregard it as a naive toy model. After a burst of interest in the 70's and the 80's, the once vivid literature about optimal foraging theory (OFT) has lost its momentum [2]. Yet, OFT and MVT have remained an active field of research in the parasitoidologists community, mostly because the sampling strategy of a parasitoid in patches of hosts and its resulting fitness gain are straightforward to evaluate, which eases both experimental and theoretical investigations [3].
This preprint [4] is in line with the long-established literature on OFT. It follows two theoretical articles [5,6] in which Vincent Calcagno and co-authors assessed the effect of changes in the environmental conditions on optimal foraging strategy. This time, they did not modify the shape of the gain function (describing the diminishing return of the cumulative intake as a function of the residency time in a patch) but the relative frequencies of good and bad patches. At first sight, that sounds like a minor modification of their earlier models. Actually, even the authors initially were fooled by the similarities before spotting the pitfalls. Here, they genuinely point out the erroneous verbal prediction in their previous paper in which some non-trivial effects of the change in patch frequencies have been overlooked. The present study indeed provides a striking example of ecological fallacy, and more specifically of Simpson's paradox which occurs when the aggregation of subgroups modifies the apparent pattern at the scale of the entire population [7,8]. In the case of MVT under constraints of habitat conversion, the increase of the residency times in both bad and good patches can result in a decrease of the average residency time at the level of the population. This apparently counter-intuitive property can be observed, for instance, when the proportion of bad quality patches strongly increases, which increases the probability that the individual forages on theses quickly exploited patches, and thus decreases its average residency time on the long run.
The authors thus put the model on the drawing board again. Proper assessment of the effect of change in the frequency of patch quality is more mathematically challenging than when one considers only changes in the shape of the gain function. The expected gain must be evaluated at the scale of the entire habitat instead of single patch. Overall, this study, which is based on a rigorous formalism, stands out as a warning against too rapid interpretations of theoretical outputs. It is not straightforward to generalize the predictions of previous models without careful evaluating their underlying hypotheses. The devil is in the details: some slight, seemingly minor, adjustments of the assumptions may have some major consequences.
The authors discussed the general conditions leading to changes in residency times or movement rates. Yet, it is worth pointing out again that it would be a mistake to blindly consider these theoretical results as forecasts for the foragers' behaviour in natura. OFT models has for a long time been criticized for sweeping under the carpet the key questions of the evolutionary dynamics and the maintenance of the optimal strategy in a population [9,10]. The distribution of available options is susceptible to change rapidly due to modifications of the environmental conditions or, even more simply, the presence of competitors which continuously remove the best options from the pool of available options [11]. The key point here is that the constant monitoring of available options implies cognitive (neural tissue is one of the most metabolically expensive tissues) and ecological costs: assessment and adjustment to the environmental conditions requires time, energy, and occasional mistakes (cost of naiveté, [12]). While rarely considered in optimal analyses, these costs should severely constraint the evolution of the subtle decision rules. Under rapidly fluctuating conditions, it could be more profitable to maintain a sub-optimal strategy (but performing reasonably well on the long run) than paying the far from negligible costs implied by the pursuit of optimal strategies [13,14]. For instance, in the analysis presented in this preprint, it is striking how close the fitness gains of the plastic and the non-plastic forager are, particularly if one remembers that the last-mentioned cognitive and ecological costs have been neglected in these calculations.
Yet, even if one can arguably question its descriptive value, such models are worth more than a cursory glance. They still have normative value insofar that they provide upper bounds for the response to modifications of the environmental conditions. Such insights are precious to design future experiments on the question. Being able to compare experimentally measured behaviours with the extremes of the null model (stubborn non-plastic forager) and the optimal strategy (only achievable by an omniscient daemon) informs about the cognitive bias or ecological costs experienced by real life foragers. I thus consider that this model, and more generally most OFT models, are still a valuable framework which deserves further examination.
References
[1] Fawcett, T. W. & Higginson, A. D. 2012 Heavy use of equations impedes communication among biologists. Proc. Natl. Acad. Sci. 109, 11735–11739. doi: 10.1073/pnas.1205259109
[2] Owens, I. P. F. 2006 Where is behavioural ecology going? Trends Ecol. Evol. 21, 356–361. doi: 10.1016/j.tree.2006.03.014
[3] Louâpre, P., Fauvergue, X., van Baaren, J. & Martel, V. 2015 The male mate search: an optimal foraging issue? Curr. Opin. Insect Sci. 9, 91–95. doi: 10.1016/j.cois.2015.02.012
[4] Calcagno, V., Hamelin, F., Mailleret, L., & Grognard, F. (2018). How optimal foragers should respond to habitat changes? On the consequences of habitat conversion. bioRxiv, 273557, ver. 4 peer-reviewed and recommended by PCI Ecol. doi: 10.1101/273557
[5] Calcagno, V., Grognard, F., Hamelin, F. M., Wajnberg, É. & Mailleret, L. 2014 The functional response predicts the effect of resource distribution on the optimal movement rate of consumers. Ecol. Lett. 17, 1570–1579. doi: 10.1111/ele.12379
[6] Calcagno, V., Mailleret, L., Wajnberg, É. & Grognard, F. 2013 How optimal foragers should respond to habitat changes: a reanalysis of the Marginal Value Theorem. J. Math. Biol. 69, 1237–1265. doi: 10.1007/s00285-013-0734-y
[7] Galipaud, M., Bollache, L., Wattier, R., Dechaume-Moncharmont, F.-X. & Lagrue, C. 2015 Overestimation of the strength of size-assortative pairing in taxa with cryptic diversity: a case of Simpson's paradox. Anim. Behav. 102, 217–221. doi: 10.1016/j.anbehav.2015.01.032
[8] Kievit, R. A., Frankenhuis, W. E., Waldorp, L. J. & Borsboom, D. 2013 Simpson's paradox in psychological science: a practical guide. Front. Psychol. 4, 513. doi: 10.3389/fpsyg.2013.00513
[9] Bolduc, J.-S. & Cézilly, F. 2012 Optimality modelling in the real world. Biol. Philos. 27, 851–869. doi: 10.1007/s10539-012-9333-3
[10] Pierce, G. J. & Ollason, J. G. 1987 Eight reasons why optimal foraging theory is a complete waste of time. Oikos 49, 111–118. doi: 10.2307/3565560
[11] Dechaume-Moncharmont, F.-X., Brom, T. & Cézilly, F. 2016 Opportunity costs resulting from scramble competition within the choosy sex severely impair mate choosiness. Anim. Behav. 114, 249–260. doi: 10.1016/j.anbehav.2016.02.019
[12] Snell-Rood, E. C. 2013 An overview of the evolutionary causes and consequences of behavioural plasticity. Anim. Behav. 85, 1004–1011. doi: 10.1016/j.anbehav.2012.12.031
[13] Fawcett, T. W., Fallenstein, B., Higginson, A. D., Houston, A. I., Mallpress, D. E. W., Trimmer, P. C. & McNamara, J. M. 2014 The evolution of decision rules in complex environments. Trends Cogn. Sci. 18, 153–161. doi: 10.1016/j.tics.2013.12.012
[14] Marshall, J. A. R., Trimmer, P. C., Houston, A. I. & McNamara, J. M. 2013 On evolutionary explanations of cognitive biases. Trends Ecol. Evol. 28, 469-473. doi: 10.1016/j.tree.2013.05.013
| How optimal foragers should respond to habitat changes? On the consequences of habitat conversion. | Vincent Calcagno, Frederic Hamelin, Ludovic Mailleret, Frederic Grognard | The Marginal Value Theorem (MVT) provides a framework to predict how habitat modifications related to the distribution of resources over patches should impact the realized fitness of individuals and their optimal rate of movement (or patch residen... |  | Behaviour & Ethology, Dispersal & Migration, Foraging, Landscape ecology, Spatial ecology, Metacommunities & Metapopulations, Theoretical ecology | Francois-Xavier Dechaume-Moncharmont | | 2018-03-05 10:42:11 | View |
When higher carrying capacities lead to faster propagation
Marjorie Haond, Thibaut Morel-Journel, Eric Lombaert, Elodie Vercken, Ludovic Mailleret & Lionel Roques
https://doi.org/10.1101/307322
When the dispersal of the many outruns the dispersal of the few
Recommended by Matthieu Barbier based on reviews by Yuval Zelnik and 1 anonymous reviewer based on reviews by Yuval Zelnik and 1 anonymous reviewer
Are biological invasions driven by a few pioneers, running ahead of their conspecifics? Or are these pioneers constantly being caught up by, and folded into, the larger flux of propagules from the established populations behind them?
In ecology and beyond, these two scenarios are known as "pulled" and "pushed" fronts, and they come with different expectations. In a pushed front, invasion speed is not just a matter of how good individuals are at dispersing and settling new locations. It becomes a collective, density-dependent property of population fluxes. And in particular, it can depend on the equilibrium abundance of the established populations inside the range, i.e. the species’ carrying capacity K, factoring in its abiotic environment and biotic interactions.
This realization is especially important because it can flip around our expectations about which species expand fast, and how to manage them. We tend to think of initial colonization and long-term abundance as two independent axes of variation among species or indeed as two ends of a spectrum, in the classic competition-colonization tradeoff [1]. When both play into invasion speed, good dispersers might not outrun good competitors. This is useful knowledge, whether we want to contain an invasion or secure a reintroduction.
In their study "When higher carrying capacities lead to faster propagation", Haond et al [2] combine mathematical analysis, Individual-Based simulations and experiments to show that various mechanisms can cause pushed fronts, whose speed increases with the carrying capacity K of the species. Rather than focus on one particular angle, the authors endeavor to demonstrate that this qualitative effect appears again and again in a variety of settings.
It is perhaps surprising that this notable and general connection between K and invasion speed has managed to garner so little fame in ecology. A large fraction of the literature employs the venerable Fisher-KPP reaction-diffusion model, which combines local logistic growth with linear diffusion in space. This model has prompted both considerable mathematical developments [3] and many applications to modelling real invasions [4]. But it only allows pulled fronts, driven by the small populations at the edge of a species range, with a speed that depends only on their initial growth rate r.
This classic setup is, however, singular in many ways. Haond et al [2] use it as a null model, and introduce three mechanisms or factors that each ensure a role of K in invasion speed, while giving less importance to the pioneers at the border.
Two factors, the Allee effect and demographic stochasticity, make small edge populations slower to grow or less likely to survive. These two factors are studied theoretically, and to make their claims stronger, the authors stack the deck against K. When generalizing equations or simulations beyond the null case, it is easy to obtain functional forms where the parameter K does not only play the role of equilibrium carrying capacity, but also affects dynamical properties such as the maximum or mean growth rate. In that case, it can trivially change the propagation speed, without it meaning anything about the role of established populations behind the front. Haond et al [2] avoid this pitfall by disentangling these effects, at the cost of slightly more peculiar expressions, and show that varying essentially nothing but the carrying capacity can still impact the speed of the invasion front.
The third factor, density-dependent dispersal, makes small populations less prone to disperse. It is well established empirically and theoretically that various biological mechanisms, from collective organization to behavioral switches, can prompt organisms in denser populations to disperse more, e.g. in such a way as to escape competition [5]. The authors demonstrate how this effect induces a link between carrying capacity and invasion speed, both theoretically and in a dispersal experiment on the parasitoid wasp, Trichogramma chilonis.
Overall, this study carries a simple and clear message, supported by valuable contributions from different angles. Although some sections are clearly written for the theoretical ecology crowd, this article has something for everyone, from the stray physicist to the open-minded manager. The collaboration between theoreticians and experimentalists, while not central, is worthy of note. Because the narrative of this study is the variety of mechanisms that can lead to the same qualitative effect, the inclusion of various approaches is not a gimmick, but helps drive home its main message. The work is fairly self-contained, although one could always wish for further developments, especially in the direction of more quantitative testing of these mechanisms.
In conclusion, Haond et al [2] effectively convey the widely relevant message that, for some species, invading is not just about the destination, it is about the many offspring one makes along the way.
References
[1] Levins, R., & Culver, D. (1971). Regional Coexistence of Species and Competition between Rare Species. Proceedings of the National Academy of Sciences, 68(6), 1246–1248. doi: 10.1073/pnas.68.6.1246
[2] Haond, M., Morel-Journel, T., Lombaert, E., Vercken, E., Mailleret, L., & Roques, L. (2018). When higher carrying capacities lead to faster propagation. BioRxiv, 307322. doi: 10.1101/307322
[3] Crooks, E. C. M., Dancer, E. N., Hilhorst, D., Mimura, M., & Ninomiya, H. (2004). Spatial segregation limit of a competition-diffusion system with Dirichlet boundary conditions. Nonlinear Analysis: Real World Applications, 5(4), 645–665. doi: 10.1016/j.nonrwa.2004.01.004
[4] Shigesada, N., & Kawasaki, K. (1997). Biological Invasions: Theory and Practice. Oxford University Press, UK.
[5] Matthysen, E. (2005). Density-dependent dispersal in birds and mammals. Ecography, 28(3), 403–416. doi: 10.1111/j.0906-7590.2005.04073.x
| When higher carrying capacities lead to faster propagation | Marjorie Haond, Thibaut Morel-Journel, Eric Lombaert, Elodie Vercken, Ludovic Mailleret & Lionel Roques | <p>This preprint has been reviewed and recommended by Peer Community In Ecology (https://dx.doi.org/10.24072/pci.ecology.100004). Finding general patterns in the expansion of natural populations is a major challenge in ecology and invasion biology... | 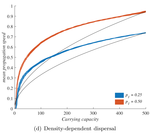 | Biological invasions, Colonization, Dispersal & Migration, Experimental ecology, Population ecology, Spatial ecology, Metacommunities & Metapopulations, Theoretical ecology | Matthieu Barbier | Yuval Zelnik | 2018-04-25 10:18:48 | View |
The return of the trophic chain: fundamental vs realized interactions in a simple arthropod food web
Inmaculada Torres-Campos, Sara Magalhães, Jordi Moya-Laraño, Marta Montserrat
https://doi.org/10.1101/324178
From deserts to avocado orchards - understanding realized trophic interactions in communities
Recommended by Francis John Burdon based on reviews by Owen Petchey and 2 anonymous reviewers
The late eminent ecologist Gary Polis once stated that “most catalogued food-webs are oversimplified caricatures of actual communities” and are “grossly incomplete representations of communities in terms of both diversity and trophic connections.” Not content with that damning indictment, he went further by railing that “theorists are trying to explain phenomena that do not exist” [1]. The latter critique might have been push back for Robert May´s ground-breaking but ultimately flawed research on the relationship between food-web complexity and stability [2]. Polis was a brilliant ecologist, and his thinking was clearly influenced by his experiences researching desert food webs. Those food webs possess an uncommon combination of properties, such as frequent omnivory, cannibalism, and looping; high linkage density (L/S); and a nearly complete absence of apex consumers, since few species completely lack predators or parasites [3]. During my PhD studies, I was lucky enough to visit Joshua Tree National Park on the way to a conference in New England, and I could immediately see the problems posed by desert ecosystems. At the time, I was ruminating on the “harsh-benign” hypothesis [4], which predicts that the relative importance of abiotic and biotic forces should vary with changes in local environmental conditions (from harsh to benign). Specifically, in more “harsh” environments, abiotic factors should determine community composition whilst weakening the influence of biotic interactions. However, in the harsh desert environment I saw first-hand evidence that species interactions were not diminished; if anything, they were strengthened. Teddy-bear chollas possessed murderously sharp defenses to protect precious water, creosote bushes engaged in belowground “chemical warfare” (allelopathy) to deter potential competitors, and rampant cannibalism amongst scorpions drove temporal and spatial ontogenetic niche partitioning. Life in the desert was hard, but you couldn´t expect your competition to go easy on you.
If that experience colored my thinking about nature’s reaction to a capricious environment, then the seminal work by Robert Paine on the marine rocky shore helped further cement the importance of biotic interactions. The insights provided by Paine [5] brings us closer to the research reported in the preprint “The return of the trophic chain: fundamental vs realized interactions in a simple arthropod food web” [6], given that the authors in that study hold the environment constant and test the interactions between different permutations of a simple community. Paine [5] was able to elegantly demonstrate using the chief protagonist Pisaster ochraceus (a predatory echinoderm also known as the purple sea star) that a keystone consumer could exert strong top-down control that radically reshaped the interactions amongst other community members. What was special about this study was that the presence of Pisaster promoted species diversity by altering competition for space by sedentary species, providing a rare example of an ecological network experiment combining trophic and non-trophic interactions. Whilst there are increasing efforts to describe these interactions (e.g., competition and facilitation) in multiplex networks [7], the authors of “The return of the trophic chain: fundamental vs realized interactions in a simple arthropod food web” [6] have avoided strictly competitive interactions for the sake of simplicity. They do focus on two trophic forms of competition, namely intraguild predation and apparent competition. These two interaction motifs, along with prey switching are relevant to my own research on the influence of cross-ecosystem prey subsidies to receiving food webs [8]. In particular, the apparent competition motif may be particularly important in the context of emergent adult aquatic insects as prey subsidies to terrestrial consumers. This was demonstrated by Henschel et al. [9] where the abundances of emergent adult aquatic midges in riparian fields adjacent to a large river helped stimulate higher abundances of spiders and lower abundances of herbivorous leafhoppers, leading to a trophic cascade. The aquatic insects had a bottom-up effect on spiders and this subsidy facilitated a top-down effect that cascaded from spiders to leafhoppers to plants. The apparent competition motif becomes relevant because the aquatic midges exerted a negative indirect effect on leafhoppers mediated through their common arachnid predators.
In the preprint “The return of the trophic chain: fundamental vs realized interactions in a simple arthropod food web” [6], the authors have described different permutations of a simple mite community present in avocado orchards (Persea americana). This community comprises of two predators (Euseius stipulatus and Neoseiulus californicus), one herbivore as shared prey (Oligonychus perseae), and pollen of Carpobrotus edulis as alternative food resource, with the potential for the intraguild predation and apparent competition interaction motifs to be expressed. The authors determined that these motifs should be realized based off pairwise feeding trials. It is common for food-web researchers to depict potential food webs, which contain all species sampled and all potential trophic links based on laboratory feeding trials (as demonstrated here) or from observational data and literature reviews [10]. In reality, not all these potential feeding links are realized because species may partition space and time, thus driving alternative food-web architectures. In “The return of the trophic chain: fundamental vs realized interactions in a simple arthropod food web” [6], the authors are able to show that placing species in combinations that should yield more complex interaction motifs based off pairwise feeding trials fails to deliver – the predators revert to their preferred prey resulting in modular and simple trophic chains to be expressed. Whilst these realized interaction motifs may be stable, there might also be a tradeoff with function by yielding less top-down control than desirable when considering the potential for ecosystem services such as pest management. These are valuable insights, although it should be noted that here the fundamental niche is described in a strictly Eltonian sense as a trophic role [11]. Adding additional niche dimensions (sensu [12]), such as a thermal gradient could alter the observed interactions, although it might be possible to explain these contingencies through metabolic and optimal foraging theory combined with species traits. Nonetheless, the results of these experiments further demonstrate the need for ecologists to cross-validate theory with empirical approaches to develop more realistic and predictive food-web models, lest they invoke the wrath of Gary Polis´ ghost by “trying to explain phenomena that do not exist”.
References
[1] Polis, G. A. (1991). Complex trophic interactions in deserts: an empirical critique of food-web theory. The American Naturalist, 138(1), 123-155. doi: 10.1086/285208
[2] May, R. M. (1973). Stability and complexity in model ecosystems. Princeton University Press, Princeton, NJ, USA
[3] Dunne, J. A. (2006). The network structure of food webs. In Pascual, M., & Dunne, J. A. (eds) Ecological Networks: Linking Structure to Dynamics in Food Webs. Oxford University Press, New York, USA, 27-86
[4] Menge, B. A., & Sutherland, J. P. (1976). Species diversity gradients: synthesis of the roles of predation, competition, and temporal heterogeneity. The American Naturalist, 110(973), 351-369. doi: 10.1086/283073
[5] Paine, R. T. (1966). Food web complexity and species diversity. The American Naturalist, 100(910), 65-75. doi: 10.1086/282400
[6] Torres-Campos, I., Magalhães, S., Moya-Laraño, J., & Montserrat, M. (2018). The return of the trophic chain: fundamental vs realized interactions in a simple arthropod food web. bioRxiv, 324178, ver. 5 peer-reviewed and recommended by PCI Ecol. doi: 10.1101/324178
[7] Kéfi, S., Berlow, E. L., Wieters, E. A., Joppa, L. N., Wood, S. A., Brose, U., & Navarrete, S. A. (2015). Network structure beyond food webs: mapping non‐trophic and trophic interactions on Chilean rocky shores. Ecology, 96(1), 291-303. doi: 10.1890/13-1424.1
[8] Burdon, F. J., & Harding, J. S. (2008). The linkage between riparian predators and aquatic insects across a stream‐resource spectrum. Freshwater Biology, 53(2), 330-346. doi: 10.1111/j.1365-2427.2007.01897.x
[9] Henschel, J. R., Mahsberg, D., & Stumpf, H. (2001). Allochthonous aquatic insects increase predation and decrease herbivory in river shore food webs. Oikos, 93(3), 429-438. doi: 10.1034/j.1600-0706.2001.930308.x
[10] Brose, U., Pavao-Zuckerman, M., Eklöf, A., Bengtsson, J., Berg, M. P., Cousins, S. H., Mulder, C., Verhoef, H. A., & Wolters, V. (2005). Spatial aspects of food webs. In de Ruiter, P., Wolters, V., Moore, J. C., & Melville-Smith, K. (eds) Dynamic Food Webs. vol 3. Academic Press, Burlington, 463-469
[11] Elton, C. (1927). Animal Ecology. Sidgwick and Jackson, London, UK
[12] Hutchinson, G. E. (1957). Concluding Remarks. Cold Spring Harbor Symposia on Quantitative Biology, 22, 415-427. doi: 10.1101/sqb.1957.022.01.039
| The return of the trophic chain: fundamental vs realized interactions in a simple arthropod food web | Inmaculada Torres-Campos, Sara Magalhães, Jordi Moya-Laraño, Marta Montserrat | <p>The mathematical theory describing small assemblages of interacting species (community modules or motifs) has proved to be essential in understanding the emergent properties of ecological communities. These models use differential equations to ... | 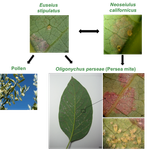 | Community ecology, Experimental ecology | Francis John Burdon | | 2018-05-16 19:34:10 | View |
Parasite intensity is driven by temperature in a wild bird
Adèle Mennerat, Anne Charmantier, Sylvie Hurtrez-Boussès, Philippe Perret, Marcel M Lambrechts
https://doi.org/10.1101/323311
The global change of species interactions
Recommended by Jan Hrcek based on reviews by 2 anonymous reviewers
What kinds of studies are most needed to understand the effects of global change on nature? Two deficiencies stand out: lack of long-term studies [1] and lack of data on species interactions [2]. The paper by Mennerat and colleagues [3] is particularly valuable because it addresses both of these shortcomings. The first one is obvious. Our understanding of the impact of climate on biota improves with longer times series of observations. Mennerat et al. [3] analysed an impressive 18-year series from multiple sites to search for trends in parasitism rates across a range of temperatures. The second deficiency (lack of species interaction data) is perhaps not yet fully appreciated, despite studies pointing this out ten years ago [2,4]. The focus is often on species range limits and how taking species interactions into account changes species range predictions based on climate alone (climate envelope models; [5]). But range limits are not everything, as the function of a species (or community, network, etc.) ultimately depends on the strengths of species interactions and not only on the presence or absence of a given species [2,4]. Mennerat et al. [3] show that in the case of birds and their nest parasites, it is the strength of the interaction that has changed, while the species involved stayed the same. Mennerat et al. [3] found nest parasitism to increase with temperature at the nestling stage. They have also searched for trends of parasitism dynamics dependence on the host, but did not find any, probably because the nest parasites are generalists and attack other bird species within the study sites. This study thus draws attention to wider networks of interacting species, and we urgently need more data to predict how interaction networks will rewire with progressing environmental change [6,7].
References
[1] Lindenmayer, D.B., Likens, G.E., Andersen, A., Bowman, D., Bull, C.M., Burns, E., et al. (2012). Value of long-term ecological studies. Austral Ecology, 37(7), 745–57. doi: 10.1111/j.1442-9993.2011.02351.x
[2] Tylianakis, J.M., Didham, R.K., Bascompte, J. & Wardle, D.A. (2008). Global change and species interactions in terrestrial ecosystems. Ecology Letters, 11(12), 1351–63. doi: 10.1111/j.1461-0248.2008.01250.x
[3] Mennerat, A., Charmantier, A., Hurtrez-Bousses, S., Perret, P. & Lambrechts, M.M. (2019). Parasite intensity is driven by temperature in a wild bird. bioRxiv, 323311. Ver. 4 peer-reviewed and recommended by PCI Ecology. doi: 10.1101/323311
[4] Gilman, S.E., Urban, M.C., Tewksbury, J., Gilchrist, G.W. & Holt, R.D. (2010). A framework for community interactions under climate change. Trends in Ecology & Evolution, 25(6), 325–31. doi: 10.1016/j.tree.2010.03.002
[5] Louthan, A.M., Doak, D.F. & Angert, A.L. (2015). Where and when do species interactions set range limits? Trends in Ecology & Evolution, 30(12), 780–92. doi: 10.1016/j.tree.2015.09.011
[6] Bartley, T.J., McCann, K.S., Bieg, C., Cazelles, K., Granados, M., Guzzo, M.M., et al. (2019). Food web rewiring in a changing world. Nature Ecology & Evolution, 3(3), 345–54. doi: 10.1038/s41559-018-0772-3
[7] Staniczenko, P.P.A., Lewis, O.T., Jones, N.S. & Reed-Tsochas, F. (2010). Structural dynamics and robustness of food webs. Ecology Letters, 13(7), 891–9. doi: 10.1111/j.1461-0248.2010.01485.x
| Parasite intensity is driven by temperature in a wild bird | Adèle Mennerat, Anne Charmantier, Sylvie Hurtrez-Boussès, Philippe Perret, Marcel M Lambrechts | <p>Increasing awareness that parasitism is an essential component of nearly all aspects of ecosystem functioning, as well as a driver of biodiversity, has led to rising interest in the consequences of climate change in terms of parasitism and dise... |  | Climate change, Evolutionary ecology, Host-parasite interactions, Parasitology, Zoology | Jan Hrcek | | 2018-05-17 14:37:14 | View |
Does elevated parasite richness in the environment affect daily path length of animals or is it the converse? An answer bringing some new elements of discussion
Recommended by Cédric Sueur based on reviews by 2 anonymous reviewers based on reviews by 2 anonymous reviewers
In 2015, Brockmeyer et al. [1] suggested that mandrills (Mandrillus sphinx) may accept additional ranging costs to avoid heavily parasitized areas. Following this paper, Bicca-Marques and Calegaro-Marques [2] questioned this interpretation and presented other hypotheses. To summarize, whilst Brockmeyer et al. [1] proposed that elevated daily path length may be a consequence of elevated parasite richness, Bicca-Marques and Calegaro-Marques [2] viewed it as a cause. In this current paper, Charpentier and Kappeler [3] respond to some of the criticisms by Bicca-Marques and Calegaro-Marques and discuss the putative parsimony of the two competing scenarios. The manuscript is interesting and focuses on an important question concerning the discussion about the social organization and home range use in wild mandrills. This answer helps to move this debate forward and should stimulate more empirical studies of the role of environmentally-transmitted parasites in shaping ranging and movement patterns of wild vertebrates. Given the elements this paper brings to the topics, it should have been published in American Journal of Primatology, the journal that published the two previous articles.
References
[1] Brockmeyer, T., Kappeler, P. M., Willaume, E., Benoit, L., Mboumba, S., & Charpentier, M. J. E. (2015). Social organization and space use of a wild mandrill (Mandrillus sphinx) group. American Journal of Primatology, 77(10), 1036–1048. doi: 10.1002/ajp.22439
[2] Bicca-Marques, J. C., & Calegaro-Marques, C. (2016). Ranging behavior drives parasite richness: A more parsimonious hypothesis. American Journal of Primatology, 78(9), 923–927. doi: 10.1002/ajp.22561
[3] Charpentier, M. J., & Kappeler, P. M. (2018). A reply to “Ranging Behavior Drives Parasite Richness: A More Parsimonious Hypothesis.” ArXiv:1805.08151v2 [q-Bio]. Retrieved from http://arxiv.org/abs/1805.08151
| A reply to “Ranging Behavior Drives Parasite Richness: A More Parsimonious Hypothesis” | Charpentier MJE, Kappeler PM | In a recent article, Bicca-Marques and Calegaro-Marques [2016] discussed the putative assumptions related to an interpretation we provided regarding an observed positive relationship between weekly averaged parasite richness of a group of mandrill... |  | Behaviour & Ethology, Evolutionary ecology, Foraging, Host-parasite interactions, Spatial ecology, Metacommunities & Metapopulations, Zoology | Cédric Sueur | | 2018-05-22 10:59:33 | View |
Impact of group management and transfer on individual sociality in Highland cattle (Bos Taurus)
Sebastian Sosa, Marie Pelé, Elise Debergue, Cedric Kuntz, Blandine Keller, Florian Robic, Flora Siegwalt-Baudin, Camille Richer, Amandine Ramos, Cédric Sueur
https://arxiv.org/abs/1805.11553v4
How empirical sciences may improve livestock welfare and help their management
Recommended by Marie Charpentier based on reviews by Alecia CARTER and 1 anonymous reviewer
Understanding how livestock management is a source of social stress and disturbances for cattle is an important question with potential applications for animal welfare programs and sustainable development. In their article, Sosa and colleagues [1] first propose to evaluate the effects of individual characteristics on dyadic social relationships and on the social dynamics of four groups of cattle. Using network analyses, the authors provide an interesting and complete picture of dyadic interactions among groupmates. Although shown elsewhere, the authors demonstrate that individuals that are close in age and close in rank form stronger dyadic associations than other pairs. Second, the authors take advantage of some transfers of animals between groups -for management purposes- to assess how these transfers affect the social dynamics of groupmates. Their central finding is that the identity of transferred animals is a key-point. In particular, removing offspring strongly destabilizes the social relationships of mothers while adding a bull into a group also profoundly impacts female-female social relationships, as social networks before and after transfer of these key-animals are completely different. In addition, individuals, especially the young ones, that are transferred without familiar conspecifics take more time to socialize with their new group members than individuals transferred with familiar groupmates, generating a potential source of stress. Interestingly, the authors end up their article with some thoughts on the implications of their findings for animal welfare and ethics. This study provides additional evidence that empirical science has a major role to play in providing recommendations regarding societal questions such as livestock management and animal wellbeing.
References
[1] Sosa, S., Pelé, M., Debergue, E., Kuntz, C., Keller, B., Robic, F., Siegwalt-Baudin, F., Richer, C., Ramos, A., & Sueur C. (2018). Impact of group management and transfer on individual sociality in Highland cattle (Bos Taurus). arXiv:1805.11553v4 [q-bio.PE] peer-reviewed and recommended by PCI Ecol. https://arxiv.org/abs/1805.11553v4
| Impact of group management and transfer on individual sociality in Highland cattle (Bos Taurus) | Sebastian Sosa, Marie Pelé, Elise Debergue, Cedric Kuntz, Blandine Keller, Florian Robic, Flora Siegwalt-Baudin, Camille Richer, Amandine Ramos, Cédric Sueur | The sociality of cattle facilitates the maintenance of herd cohesion and synchronisation, making these species the ideal choice for domestication as livestock for humans. However, livestock populations are not self-regulated, and farmers transfer ... |  | Behaviour & Ethology, Social structure | Marie Charpentier | | 2018-05-30 14:05:39 | View |
Soil variation response is mediated by growth trajectories rather than functional traits in a widespread pioneer Neotropical tree
Sébastien Levionnois, Niklas Tysklind, Eric Nicolini, Bruno Ferry, Valérie Troispoux, Gilles Le Moguedec, Hélène Morel, Clément Stahl, Sabrina Coste, Henri Caron, Patrick Heuret
https://doi.org/10.1101/351197
Growth trajectories, better than organ-level functional traits, reveal intraspecific response to environmental variation
Recommended by François Munoz based on reviews by Georges Kunstler and François Munoz based on reviews by Georges Kunstler and François Munoz
Functional traits are “morpho-physio-phenological traits which impact fitness indirectly via their effects on growth, reproduction and survival” [1]. Most functional traits are defined at organ level, e.g. for leaves, roots and stems, and reflect key aspects of resource acquisition and resource use by organisms for their development and reproduction [2]. More rarely, some functional traits can be related to spatial development, such as vegetative height and lateral spread in plants.
Organ-level traits are especially popular because they can be measured in a standard way and easily compared over many plants. But these traits can broadly vary during the life of an organism. For instance, Roggy et al. [3] found that Leaf Mass Area can vary from 30 to 140 g.m^(-2) between seedling and adult stages for the canopy tree Dicorynia guianensis in French Guiana. Fortunel et al. [4] have also showed that developmental stages much contribute to functional trait variation within several Micropholis tree species in lowland Amazonia.
The way plants grow and invest resources into organs is variable during life and allows defining specific developmental sequences and architectural models [5,6]. There is clear ontogenic variation in leaf number, leaf properties and ramification patterns. Ontogenic variations reflect changing adaptation of an individual over its life, depending on the changing environmental conditions.
In this regard, measuring a single functional trait at organ level in adult trees should miss the variation of resource acquisition and use strategies over time. Thus we should built a more integrative approach of ecological development, also called “eco-devo” approach [7].
Although the ecological significance of ontogeny and developmental strategies is now well known, the extent to which it contributes to explain species survival and coexistence in communities is still broadly ignored in functional ecology. Levionnois et al. [8] investigated intraspecific variation of functional traits and growth trajectories in a typical, early-successional tree species in French Guiana, Amazonia. This species, Cecropia obtusa, is generalist regarding soil type and can be found on both white sand and ferralitic soil. The study examines whether there in intraspecific variation in functional traits and growth trajectories of C. obtusa in response to the contrasted soil types.
The tree communities observed on the two types of soils include species with distinctive functional trait values, that is, there are changes in species composition related to different species strategies along the classical wood and leaf economic spectra. The populations of C. obtusa found on the two soils showed some difference in functional traits, but it did not concern traits related to the main economic spectra. Conversely, the populations showed different growth strategies, in terms of spatial and temporal development.
The major lessons we can learn from the study are:
(i) Functional traits measured at organ level cannot reflect well how long-lived plants collect and invest resources during their life. The results show the potential of considering architectural and developmental traits together with organ-level functional traits, to better acknowledge the variation in ecological strategies over plant life, and thus to better understand community assembly processes.
(ii) What makes functional changes between communities differs when considering interspecific and intraspecific variation. Species turnover should encompass different corteges of soil specialists. These specialists are sorted along economic spectra, as shown in tropical rainforests and globally [2]. Conversely, a generalist species such as C. obtusa does occur on contrasted soil, which entails that it can accommodate the contrasted ecological conditions. However, the phenotypic adjustment is not related to how leaves and wood ensure photosynthesis, water and nutrient acquisition, but regards the way the resources are allocated to growth and reproduction over time.
The results of the study stress the need to better integrate growth strategies and ontogeny in the research agenda of functional ecology. We can anticipate that organ-level functional traits and growth trajectories will be more often considered together in ecological studies. The integration should help better understand the temporal niche of organisms, and how organisms can coexist in space and time with other organisms during their life. Recently, Klimešová et al. [9] have proposed standardized protocols for collecting plant modularity traits. Such effort to propose easy-to-measure traits representing plant development and ontogeny, with clear functional roles, should foster the awaited development of an “eco-devo” approach.
References
[1] Violle, C., Navas, M. L., Vile, D., Kazakou, E., Fortunel, C., Hummel, I., & Garnier, E. (2007). Let the concept of trait be functional!. Oikos, 116(5), 882-892. doi: 10.1111/j.0030-1299.2007.15559.x
[2] Díaz, S. et al. (2016). The global spectrum of plant form and function. Nature, 529(7585), 167-171. doi: 10.1038/nature16489
[3] Roggy, J. C., Nicolini, E., Imbert, P., Caraglio, Y., Bosc, A., & Heuret, P. (2005). Links between tree structure and functional leaf traits in the tropical forest tree Dicorynia guianensis Amshoff (Caesalpiniaceae). Annals of forest science, 62(6), 553-564. doi: 10.1051/forest:2005048
[4] Fortunel, C., Stahl, C., Heuret, P., Nicolini, E. & Baraloto, C. (2020). Disentangling the effects of environment and ontogeny on tree functional dimensions for congeneric species in tropical forests. New Phytologist. doi: 10.1111/nph.16393
[5] Barthélémy, D., & Caraglio, Y. (2007). Plant architecture: a dynamic, multilevel and comprehensive approach to plant form, structure and ontogeny. Annals of botany, 99(3), 375-407. doi: 10.1093/aob/mcl260
[6] Hallé, F., & Oldeman, R. A. (1975). An essay on the architecture and dynamics of growth of tropical trees. Kuala Lumpur: Penerbit Universiti Malaya.
[7] Sultan, S. E. (2007). Development in context: the timely emergence of eco-devo. Trends in Ecology & Evolution, 22(11), 575-582. doi: 10.1016/j.tree.2007.06.014
[8] Levionnois, S., Tysklind, N., Nicolini, E., Ferry, B., Troispoux, V., Le Moguedec, G., Morel, H., Stahl, C., Coste, S., Caron, H. & Heuret, P. (2020). Soil variation response is mediated by growth trajectories rather than functional traits in a widespread pioneer Neotropical tree. bioRxiv, 351197, ver. 4 peer-reviewed and recommended by PCI Ecology. doi: 10.1101/351197
[9] Klimešová, J. et al. (2019). Handbook of standardized protocols for collecting plant modularity traits. Perspectives in Plant Ecology, Evolution and Systematics, 40, 125485. doi: 10.1016/j.ppees.2019.125485
| Soil variation response is mediated by growth trajectories rather than functional traits in a widespread pioneer Neotropical tree | Sébastien Levionnois, Niklas Tysklind, Eric Nicolini, Bruno Ferry, Valérie Troispoux, Gilles Le Moguedec, Hélène Morel, Clément Stahl, Sabrina Coste, Henri Caron, Patrick Heuret | <p style="text-align: justify;">1- Trait-environment relationships have been described at the community level across tree species. However, whether interspecific trait-environment relationships are consistent at the intraspecific level is yet unkn... |  | Botany, Eco-evolutionary dynamics, Habitat selection, Ontogeny, Tropical ecology | François Munoz | | 2018-06-21 17:13:17 | View |
Recommendations to address uncertainties in environmental risk assessment using toxicokinetics-toxicodynamics models
Virgile Baudrot and Sandrine Charles
https://doi.org/10.1101/356469
Addressing uncertainty in Environmental Risk Assessment using mechanistic toxicological models coupled with Bayesian inference
Recommended by Luis Schiesari based on reviews by Andreas Focks and 2 anonymous reviewers
Environmental Risk Assessment (ERA) is a strategic conceptual framework to characterize the nature and magnitude of risks, to humans and biodiversity, of the release of chemical contaminants in the environment. Several measures have been suggested to enhance the science and application of ERA, including the identification and acknowledgment of uncertainties that potentially influence the outcome of risk assessments, and the appropriate consideration of temporal scale and its linkage to assessment endpoints [1].
Baudrot & Charles [2] proposed to approach these questions by coupling toxicokinetics-toxicodynamics models, which describe the time-course of processes leading to the adverse effects of a toxicant, with Bayesian inference. TKTD models separate processes influencing an organismal internal exposure (´toxicokinetics´, i.e., the uptake, bioaccumulation, distribution, biotransformation and elimination of a toxicant) from processes leading to adverse effects and ultimately its death (´toxicodynamics´) [3]. Although species and substance specific, the mechanistic nature of TKTD models facilitates the comparison of different toxicants, species, life stages, environmental conditions and endpoints [4].
Baudrot & Charles [2] investigated the use of a Bayesian framework to assess the uncertainties surrounding the calibration of General Unified Threshold Models of Survival (a category of TKTD) with data from standard toxicity tests, and their propagation to predictions of regulatory toxicity endpoints such as LC(x,t) [the lethal concentration affecting any x% of the population at any given exposure duration of time t] and MF(x,t) [an exposure multiplication factor leading to any x% effect reduction due to the contaminant at any time t].
Once calibrated with empirical data, GUTS models were used to explore individual survival over time, and under untested exposure conditions. Lethal concentrations displayed a strong curvilinear decline with time of exposure. For a given total amount of contaminant, pulses separated by short time intervals yielded higher mortality than pulses separated by long time intervals, as did few pulses of high amplitude when compared to multiple pulses of low amplitude. The response to a pulsed contaminant exposure was strongly influenced by contaminant depuration times. These findings highlight one important contribution of TKTD modelling in ecotoxicology: they represent just a few of the hundreds of exposure scenarios that could be mathematically explored, and that would be unfeasible or even unethical to conduct experimentally.
GUTS models were also used for interpolations or extrapolations of assessment endpoints, and their marginal distributions. A case in point is the incipient lethal concentration. The responses of model organisms to contaminants in standard toxicity tests are typically assessed at fixed times of exposure (e.g. 24h or 48h in the Daphnia magna acute toxicity test). However, because lethal concentrations are strongly time-dependent, it has been suggested that a more meaningful endpoint would be the incipient (i.e. asymptotic) lethal concentration when time of exposure increases to infinity. The authors present a mathematical solution for calculating the marginal distribution of such incipient lethal concentration, thereby providing both more relevant information and a way of comparing experiments, compounds or species tested for different periods of time.
Uncertainties were found to change drastically with time of exposure, being maximal at extreme values of x for both LC(x,t) and MF(x,t). In practice this means that assessment endpoints estimated when the effects of the contaminant are weak (such as LC10, the contaminant concentration resulting in the mortality of 10% of the experimental population), a commonly used assessment value in ERA, are prone to be highly variable.
The authors end with recommendations for improved experimental design, including (i) using assessment endpoints at intermediate values of x (e.g., LC50 instead of LC10) (ii) prolonging exposure and recording mortality over the course of the experiment (iii) experimenting one or few peaks of high amplitude close to each other when assessing pulsed exposure. Whereas these recommendations are not that different from current practices, they are based on a more coherent mechanistic grounding.
Overall, this and other contributions from Charles, Baudrot and their research group contribute to turn TKTD models into a real tool for Environmental Risk Assessment. Further enhancement of ERA´s science and application could be achieved by extending the use of TKTD models to sublethal rather than lethal effects, and to chronic rather than acute exposure, as these are more controversial issues in decision-making regarding contaminated sites.
References
[1] Dale, V. H., Biddinger, G. R., Newman, M. C., Oris, J. T., Suter, G. W., Thompson, T., ... & Chapman, P. M. (2008). Enhancing the ecological risk assessment process. Integrated environmental assessment and management, 4(3), 306-313. doi: 10.1897/IEAM_2007-066.1
[2] Baudrot, V., & Charles, S. (2018). Recommendations to address uncertainties in environmental risk assessment using toxicokinetics-toxicodynamics models. bioRxiv, 356469, ver. 3 peer-reviewed and recommended by PCI Ecol. doi: 10.1101/356469
[3] EFSA Panel on Plant Protection Products and their Residues (PPR), Ockleford, C., Adriaanse, P., Berny, P., Brock, T., Duquesne, S., ... & Kuhl, T. (2018). Scientific Opinion on the state of the art of Toxicokinetic/Toxicodynamic (TKTD) effect models for regulatory risk assessment of pesticides for aquatic organisms. EFSA Journal, 16(8), e05377. doi: 10.2903/j.efsa.2018.5377
[4] Jager, T., Albert, C., Preuss, T. G., & Ashauer, R. (2011). General unified threshold model of survival-a toxicokinetic-toxicodynamic framework for ecotoxicology. Environmental science & technology, 45(7), 2529-2540. doi: 10.1021/es103092a
| Recommendations to address uncertainties in environmental risk assessment using toxicokinetics-toxicodynamics models | Virgile Baudrot and Sandrine Charles | <p>Providing reliable environmental quality standards (EQS) is a challenging issue for environmental risk assessment (ERA). These EQS are derived from toxicity endpoints estimated from dose-response models to identify and characterize the environm... | 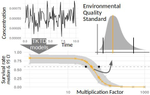 | Chemical ecology, Ecotoxicology, Experimental ecology, Statistical ecology | Luis Schiesari | | 2018-06-27 21:33:30 | View |







 based on reviews by Frederick Adler, Andrew Higginson and 1 anonymous reviewer
based on reviews by Frederick Adler, Andrew Higginson and 1 anonymous reviewer


 based on reviews by Yuval Zelnik and 1 anonymous reviewer
based on reviews by Yuval Zelnik and 1 anonymous reviewer






 based on reviews by 2 anonymous reviewers
based on reviews by 2 anonymous reviewers


 based on reviews by Georges Kunstler and François Munoz
based on reviews by Georges Kunstler and François Munoz













