Assessing metacommunity processes through signatures in spatiotemporal turnover of community composition
Franck Jabot, Fabien Laroche, Francois Massol, Florent Arthaud, Julie Crabot, Maxime Dubart, Simon Blanchet, Francois Munoz, Patrice David, Thibault Datry
https://doi.org/10.1101/480335
On the importance of temporal meta-community dynamics for our understanding of assembly processes
Recommended by Werner Ulrich based on reviews by Joaquín Hortal and 2 anonymous reviewers based on reviews by Joaquín Hortal and 2 anonymous reviewers
The processes that trigger community assembly are still in the centre of ecological interest. While prior work mostly focused on spatial patterns of co-occurrence within a meta-community framework [reviewed in 1, 2] recent studies also include temporal patterns of community composition [e.g. 3, 4, 5, 6]. In this preprint [7], Franck Jabot and co-workers extend they prior approaches to quasi neutral community assembly [8, 9, 10] and develop an analytical framework of spatial and temporal diversity turnover. A simple and heuristic path model for beta diversity and an extended ecological drift model serve as starting points. The model can be seen as a counterpart to Ulrich et al. [5]. These authors implemented competitive hierarchies into their neutral meta-community model while the present paper focuses on environmental filtering. Most important, the model and parameterization of four empirical data sets on aquatic plant and animal meta-communities used by Jabot et al. returned a consistent high influence of environmental stochasticity on species turnover. Of course, this major result does not come to a surprise. As typical for this kind of models it depends also to a good deal on the initial model settings. It nevertheless makes a strong conceptual point for the importance of environmental variability over dispersal and richness effects. One interesting side effect regards the impact of richness differences (ΔS). Jabot et al. interpret this as a ‘nuisance variable’ as they do not have a stringent explanation. Of course, it might be a pure statistical bias introduced by the Soerensen metric of turnover that is normalized by richness. However, I suspect that there is more behind the ΔS effect. Richness differences are generally associated with respective differences in total abundances and introduce source – sink dynamics that inevitably shape subsequent colonization – extinction processes. It would be interesting to see whether ΔS alone is able to trigger observed patterns of community assembly and community composition. Such an analysis would require partitioning of species turnover into richness and nestedness effects [11]. I encourage Jabot et al. to undertake such an effort.
The present paper is also another call to include temporal population variability into metapopulation models for a better understanding of the dynamics and triggering of community assembly. In a next step, competitive interactions should be included into the model to infer the relative importance of both factors.
References
[1] Götzenberger, L. et al. (2012). Ecological assembly rules in plant communities—approaches, patterns and prospects. Biological reviews, 87(1), 111-127. doi: 10.1111/j.1469-185X.2011.00187.x
[2] Ulrich, W., & Gotelli, N. J. (2013). Pattern detection in null model analysis. Oikos, 122(1), 2-18. doi: 10.1111/j.1600-0706.2012.20325.x
[3] Grilli, J., Barabás, G., Michalska-Smith, M. J., & Allesina, S. (2017). Higher-order interactions stabilize dynamics in competitive network models. Nature, 548(7666), 210. doi: 10.1038/nature23273
[4] Nuvoloni, F. M., Feres, R. J. F., & Gilbert, B. (2016). Species turnover through time: colonization and extinction dynamics across metacommunities. The American Naturalist, 187(6), 786-796. doi: 10.1086/686150
[5] Ulrich, W., Jabot, F., & Gotelli, N. J. (2017). Competitive interactions change the pattern of species co‐occurrences under neutral dispersal. Oikos, 126(1), 91-100. doi: 10.1111/oik.03392
[6] Dobramysl, U., Mobilia, M., Pleimling, M., & Täuber, U. C. (2018). Stochastic population dynamics in spatially extended predator–prey systems. Journal of Physics A: Mathematical and Theoretical, 51(6), 063001. doi: 10.1088/1751-8121/aa95c7
[7] Jabot, F., Laroche, F., Massol, F., Arthaud, F., Crabot, J., Dubart, M., Blanchet, S., Munoz, F., David, P., and Datry, T. (2019). Assessing metacommunity processes through signatures in spatiotemporal turnover of community composition. bioRxiv, 480335, ver. 3 peer-reviewed and recommended by PCI Ecology. doi: 10.1101/480335
[8] Jabot, F., & Chave, J. (2011). Analyzing tropical forest tree species abundance distributions using a nonneutral model and through approximate Bayesian inference. The American Naturalist, 178(2), E37-E47. doi: 10.1086/660829
[9] Jabot, F., & Lohier, T. (2016). Non‐random correlation of species dynamics in tropical tree communities. Oikos, 125(12), 1733-1742. doi: 10.1111/oik.03103
[10] Datry, T., Bonada, N., & Heino, J. (2016). Towards understanding the organisation of metacommunities in highly dynamic ecological systems. Oikos, 125(2), 149-159. doi: 10.1111/oik.02922
[11] Baselga, A. (2010). Partitioning the turnover and nestedness components of beta diversity. Global ecology and biogeography, 19(1), 134-143. doi: 10.1111/j.1466-8238.2009.00490.x
| Assessing metacommunity processes through signatures in spatiotemporal turnover of community composition | Franck Jabot, Fabien Laroche, Francois Massol, Florent Arthaud, Julie Crabot, Maxime Dubart, Simon Blanchet, Francois Munoz, Patrice David, Thibault Datry | <p>Although metacommunity ecology has been a major field of research in the last decades, with both conceptual and empirical outputs, the analysis of the temporal dynamics of metacommunities has only emerged recently and still consists mostly of r... | 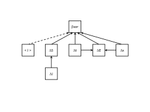 | Biodiversity, Coexistence, Community ecology, Spatial ecology, Metacommunities & Metapopulations | Werner Ulrich | | 2018-11-29 14:58:54 | View |
Blood, sweat and tears: a review of non-invasive DNA sampling
Marie-Caroline Lefort, Robert H Cruickshank, Kris Descovich, Nigel J Adams, Arijana Barun, Arsalan Emami-Khoyi, Johnaton Ridden, Victoria R Smith, Rowan Sprague, Benjamin Waterhouse, Stephane Boyer
https://doi.org/10.1101/385120
Words matter: extensive misapplication of "non-invasive" in describing DNA sampling methods, and proposed clarifying terms
Recommended by Thomas Wilson Sappington based on reviews by 2 anonymous reviewers
The ability to successfully sequence trace quantities of environmental DNA (eDNA) has provided unprecedented opportunities to use genetic analyses to elucidate animal ecology, behavior, and population structure without affecting the behavior, fitness, or welfare of the animal sampled. Hair associated with an animal track in the snow, the shed exoskeleton of an insect, or a swab of animal scat are all examples of non-invasive methods to collect eDNA. Despite the seemingly uncomplicated definition of "non-invasive" as proposed by Taberlet et al. [1], Lefort et al. [2] highlight that its appropriate application to sampling methods in practice is not so straightforward. For example, collecting scat left behind on the forest floor by a mammal could be invasive if feces is used by that species to mark territorial boundaries. Other collection strategies such as baited DNA traps to collect hair, capturing and handling an individual to swab or stimulate emission of a body fluid, or removal of a presumed non essential body part like a feather, fish scale, or even a leg from an insect are often described as "non-invasive" sampling methods. However, such methods cannot be considered truly non-invasive. At a minimum, attracting or capturing and handling an animal to obtain a DNA sample interrupts its normal behavioral routine, but additionally can cause both acute and long-lasting physiological and behavioral stress responses and other effects. Even invertebrates exhibit long-term hypersensitization after an injury, which manifests as heightened vigilance and enhanced escape responses [3-5].
Through an extensive analysis of 380 papers published from 2013-2018, Lefort et al. [2] document the widespread misapplication of the term "non-invasive" to methods used to sample DNA. An astonishing 58% of these papers employed the term incorrectly. A big part of the problem is that "non-invasive" is usually used by authors in the medical or veterinary sense of not breaking the skin or entering the body [6], rather than in the broader, ecological sense of Taberlet et al. [1]. The authors argue that correct use of the term matters, because it may lead naive readers – one can imagine students, policy makers, and the general public – to incorrectly assume a particular method is safe to use in a situation where disturbing the animal could affect experimental results or raise animal welfare concerns. Such assumptions can affect experimental design, as well as interpretations of one's own or others' data.
The importance of the Lefort et al. [2] paper lies in part on the authors' call for the research community to be much more careful when applying the term "non-invasive" to methods of DNA sampling. This call cannot be shrugged off as a minor problem in a few papers – as their literature review demonstrates, "non-invasive" is being applied incorrectly more often than not. The authors recognize that not all DNA sampling must be non-invasive to be useful or ethical. Examples include taking samples for DNA extraction from museum specimens, or opportunistically from carcasses of animals hunted either legally or seized by authorities from poachers. In many cases, there may be no viable non-invasive method to obtain DNA, but a researcher strives to collect samples using methods that, although they may involve taking a sample directly from the animal's body, do not disrupt, or only slightly disrupt behavior, fitness, or welfare of the animal. Thus, the other important contribution by Lefort et al. [2] is to propose the terms "non-disruptive" and "minimally-disruptive" to describe such sampling methods, which are not strictly non-invasive. While gray areas undoubtedly remain, as acknowledged by the authors, answering the call for correct use of "non-invasive" and applying the proposed new terms for certain types of invasive sampling with a focus on level of disruption, will go a long way in limiting misconceptions and misinterpretations caused by the current confusion in terminology.
References
[1] Taberlet P., Waits L. P. and Luikart G. 1999. Noninvasive genetic sampling: look before you leap. Trends Ecol. Evol. 14: 323-327. doi: 10.1016/S0169-5347(99)01637-7
[2] Lefort M.-C., Cruickshank R. H., Descovich K., Adams N. J., Barun A., Emami-Khoyi A., Ridden J., Smith V. R., Sprague R., Waterhouse B. R. and Boyer S. 2019. Blood, sweat and tears: a review of non-invasive DNA sampling. bioRxiv, 385120, ver. 4 peer-reviewed and recommended by PCI Ecology. doi: 10.1101/385120
[3] Khuong T. M., Wang Q.-P., Manion J., Oyston L. J., Lau M.-T., Towler H., Lin Y. Q. and Neely G. G. 2019. Nerve injury drives a heightened state of vigilance and neuropathic sensitization in Drosophila. Science Advances 5: eaaw4099. doi: 10.1126/sciadv.aaw4099
[4] Crook, R. J., Hanlon, R. T. and Walters, E. T. 2013. Squid have nociceptors that display widespread long-term sensitization and spontaneous activity after bodily injury. Journal of Neuroscience, 33(24), 10021-10026. doi: 10.1523/JNEUROSCI.0646-13.2013
[5] Walters E. T. 2018. Nociceptive biology of molluscs and arthropods: evolutionary clues about functions and mechanisms potentially related to pain. Frontiers in Physiololgy 9: doi: 10.3389/fphys.2018.01049
[6] Garshelis, D. L. 2006. On the allure of noninvasive genetic sampling-putting a face to the name. Ursus 17: 109-123. doi: 10.2192/1537-6176(2006)17[109:OTAONG]2.0.CO;2
| Blood, sweat and tears: a review of non-invasive DNA sampling | Marie-Caroline Lefort, Robert H Cruickshank, Kris Descovich, Nigel J Adams, Arijana Barun, Arsalan Emami-Khoyi, Johnaton Ridden, Victoria R Smith, Rowan Sprague, Benjamin Waterhouse, Stephane Boyer | <p>The use of DNA data is ubiquitous across animal sciences. DNA may be obtained from an organism for a myriad of reasons including identification and distinction between cryptic species, sex identification, comparisons of different morphocryptic ... | 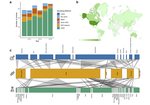 | Behaviour & Ethology, Conservation biology, Molecular ecology, Zoology | Thomas Wilson Sappington | | 2018-11-30 13:33:31 | View |
Environmental heterogeneity drives tsetse fly population dynamics and control
Cecilia H, Arnoux S, Picault S, Dicko A, Seck MT, Sall B, Bassene M, Vreysen M, Pagabeleguem S, Bance A, Bouyer J, Ezanno P
https://doi.org/10.1101/493650
Modeling jointly landscape complexity and environmental heterogeneity to envision new strategies for tsetse flies control
Recommended by Benjamin Roche based on reviews by Timothée Vergne and 1 anonymous reviewer
Today, understanding spatio-temporal dynamics of pathogens is pivotal to understand their transmission and controlling them. First, understanding this dynamics can reveal the ecology of their transmission [1]. Indeed, such knowledge, based on data that are quite easy to access, can shed light on transmission modes, which could rely on different animal species that can be spatially distributed in a non-uniform way [2]. This is especially true for pathogens with complex life-cycles, despite that investigating such dynamics is very challenging and rely mostly on mathematical models.
Moreover, this knowledge can also highlight some weak points in a complex web of transmission and therefore allowing us to envision new innovative control strategies. This has been first proposed on human pathogens, where connectivity among populations can be analyzed to identify which connections need to be targeted to stop or slow down an epidemics [3]. However, this idea is increasingly recognized as a promising new approach for pathogens involving vector populations, especially regarding the complexity to decrease on a long-term the abundance of these vector populations [4].
In "Environmental heterogeneity drives tsetse fly population dynamics and control" [5], Cecilia and co-authors have developed a sophisticated spatio-temporal mechanistic model to figure out how local environment, involved within landscape of different complexities, can impact the population dynamics of tsetse flies, an invertebrate species that can serve as a vector for many pathogens of animal and human importance. They found that spatial patches with the lowest temperature mean and the lowest environmental fluctuations can act as refuge for this species, representing therefore preferential targets for disease control.
The reviewers and I agree that the mathematical framework developed address very well an important topic for both ecological and public health literature. More importantly, it shows how fundamental ecological knowledge can drive pathogen control strategies, opening an interesting avenue for cross-disciplinary research on vector-borne diseases.
References
[1] Grenfell, B. T., Bjørnstad, O. N., & Kappey, J. (2001). Travelling waves and spatial hierarchies in measles epidemics. Nature, 414(6865), 716-723. doi: 10.1038/414716a
[2] Perkins, S. E., Cattadori, I. M., Tagliapietra, V., Rizzoli, A. P., & Hudson, P. J. (2003). Empirical evidence for key hosts in persistence of a tick-borne disease. International journal for parasitology, 33(9), 909-917. doi: 10.1016/S0020-7519(03)00128-0
[3] Colizza, V., Barrat, A., Barthélemy, M., & Vespignani, A. (2006). The role of the airline transportation network in the prediction and predictability of global epidemics. Proceedings of the National Academy of Sciences, 103(7), 2015-2020. doi: 10.1073/pnas.0510525103
[4] Pepin, K. M., Leach, C. B., Marques-Toledo, C., Laass, K. H., Paixao, K. S., et al. (2015) Utility of mosquito surveillance data for spatial prioritization of vector control against dengue viruses in three Brazilian cities. Parasites & Vectors 8, 1–15. doi: 10.1186/s13071-015-0659-y
[5] Cecilia, H., Arnoux, S., Picault, S., Dicko, A., Seck, M. T., Sall, B., Bassène, M., Vreysen, M., Pagabeleguem, S., Bancé, A., Bouyer, J. and Ezanno, P.(2019). Environmental heterogeneity drives tsetse fly population dynamics and control. bioRxiv 493650, ver. 3 peer-reviewed and recommended by PCI Ecology. doi: 10.1101/493650
| Environmental heterogeneity drives tsetse fly population dynamics and control | Cecilia H, Arnoux S, Picault S, Dicko A, Seck MT, Sall B, Bassene M, Vreysen M, Pagabeleguem S, Bance A, Bouyer J, Ezanno P | <p>A spatially and temporally heterogeneous environment may lead to unexpected population dynamics. Knowledge still is needed on which of the local environment properties favour population maintenance at larger scale. For pathogen vectors, such as... | 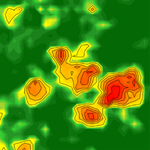 | Biological control, Population ecology, Spatial ecology, Metacommunities & Metapopulations | Benjamin Roche | | 2018-12-14 12:13:39 | View |
Environmental perturbations and transitions between ecological and evolutionary equilibria: an eco-evolutionary feedback framework
Tim Coulson
https://doi.org/10.1101/509067
Stasis and the phenotypic gambit
Recommended by Tom Van Dooren based on reviews by Jacob Johansson, Katja Räsänen and 1 anonymous reviewer
The preprint "Environmental perturbations and transitions between ecological and evolutionary equilibria: an eco-evolutionary feedback framework" by Coulson (2020) presents a general framework for evolutionary ecology, useful to interpret patterns of selection and evolutionary responses to environmental transitions. The paper is written in an accessible and intuitive manner. It reviews important concepts which are at the heart of evolutionary ecology. Together, they serve as a worldview which you can carry with you to interpret patterns in data or observations in nature. I very much appreciate it that Coulson (2020) presents his personal intuition laid bare, the framework he uses for his research and how several strong concepts from theoretical ecology fit in there. Overviews as presented in this paper are important to understand how we as researchers put the pieces together.
A main message of the paper is that resource detection and acquisition traits, broadly called "resource accrual traits" are at the core of evolutionary dynamics. These traits and the processes they are involved in often urge some degree of individual specialization. Not all traits are resource accrual traits all the time. Guppies are cited as an example, which have traits in high predation environments that make foraging easier for them, such as being less conspicuous to predators. In the absence of predators, these same traits might be neutral. Their colour pattern might then contribute much less to the odds of obtaining resources.
"Resource accrual" reminds me of discussions of resource holding potential (Parker 1974), which can be for example the capacity to remain on a bird feeder without being dislodged. However, the idea is much broader and aggression does not need to be important for the acquisition of resources. Evolutionary success is reserved for those steadily obtaining resources. This recalls the pessimization principle of Metz et al. (2008), which applies in a restricted set of situations and where the strategy which persists at the lowest resource levels systematically wins evolutionary contests. If this principle would apply universally, the world then inherently become the worst possible. Resources determine energy budgets and different life history strategies allocate these differently to maximize fitness. The fine grain of environments and the filtration by individual histories generate a lot of variation in outcomes. However, constraint-centered approaches (Kempes et al. 2019, Kooijman 2010) are mentioned but are not at the core of this preprint. Evolution is rather seen as dynamic programming optimization with interactions within and between species. Coulson thus extends life history studies such as for example Tonnabel et al. (2012) with eco-evolutionary feedbacks. Examples used are guppies, algae-rotifer interactions and others. Altogether, this makes for an optimistic paper pushing back the pessimization principle.
Populations are expected to spend most of the time in quasi-equilibrium states where the long run stochastic growth rate is close to zero for all genotypes, alleles or other chosen classes. In the preprint, attention is given to reproductive value calculus, another strong tool in evolutionary dynamics (Grafen 2006, Engen et al. 2009), which tells us how classes within a population contribute to population composition in the distant future. The expected asymptotic fitness of an individual is equated to its expected reproductive value, but this might require particular ways of calculating reproductive values (Coulson 2020). Life history strategies can also be described by per generation measures such as R0 (currently on everyone's radar due to the coronavirus pandemic), generation time etc. Here I might disagree because I believe that this focus in per generation measures can lead to an incomplete characterization of plastic and other strategies involved in strategies such as bet-hedging. A property at quasi-equilibrium states is precise enough to serve as a null hypothesis which can be falsified: all types must in the long run leave equal numbers of descendants. If there is any property in evolutionary ecology which is useful it is this one and it rightfully merits attention.
However, at quasi-equilibrium states, directional selection has been observed, often without the expected evolutionary response. The preprint aims to explain this and puts forward the presence of non-additive gene action as a mechanism. I don't believe that it is the absence of clonal inheritance which matters very much in itself (Van Dooren 2006) unless genes with major effect are present in protected polymorphisms. The preprint remains a bit unclear on how additive gene action is broken, and here I add from the sphere in which I operate. Non-additive gene action can be linked to non-linear genotype-phenotype maps (Van Dooren 2000, Gilchrist and Nijhout 2001) and if these maps are non-linear enough to create constraints on phenotype determination, by means of maximum or minimum phenotypes which cannot be surpassed for any combination of the underlying traits, then they create additional evolutionary quasi-equilibrium states, with directional selection on a phenotype such as body size. I believe Coulson hints at this option (Coulson et al. 2006), but also at a different one: if body size is mostly determined by variation in resource accrual traits, then the resource accrual traits can be under stabilizing selection while body size is not. This requires that all resource accrual traits affect other phenotypic or demographic properties next to body size. In both cases, microevolutionary outcomes cannot be inferred from inspecting body sizes alone, either resource accrual traits need to be included explicitly, or non-linearities, or both when the map between resource accrual and body size is non-linear (Van Dooren 2000).
The discussion of the phenotypic gambit (Grafen 1984) leads to another long-standing issue in evolutionary biology. Can predictions of adaptation be made by inspecting and modelling individual phenotypes alone? I agree that with strongly non-linear genotype-phenotype maps they cannot and for multivariate sets of traits, genetic and phenotypic correlations can be very different (Hadfield et al. 2007). However, has the phenotypic gambit ever claimed to be valid globally or should it rather be used locally for relatively small amounts of variation? Grafen (1984) already contained caveats which are repeated here. As a first approximation, additivity might produce quite correct predictions and thus make the gambit operational in many instances. When important individual traits are omitted, it may just be misspecified. I am interested to see cases where the framework Coulson (2020) proposes is used for very large numbers of phenotypic and genotypic attributes. In the end, these highly dimensional trait distributions might basically collapse to a few major axes of variation due to constraints on resource accrual.
I highly recommend reading this preprint and I am looking forward to the discussion it will generate.
References
[1] Coulson, T. (2020) Environmental perturbations and transitions between ecological and evolutionary equilibria: an eco-evolutionary feedback framework. bioRxiv, 509067, ver. 4 peer-reviewed and recommended by PCI Ecology. doi: 10.1101/509067
[2] Coulson, T., Benton, T. G., Lundberg, P., Dall, S. R. X., and Kendall, B. E. (2006). Putting evolutionary biology back in the ecological theatre: a demographic framework mapping genes to communities. Evolutionary Ecology Research, 8(7), 1155-1171.
[3] Engen, S., Lande, R., Sæther, B. E. and Dobson, F. S. (2009) Reproductive value and the stochastic demography of age-structured populations. The American Naturalist 174: 795-804. doi: 10.1086/647930
[4] Gilchrist, M. A. and Nijhout, H. F. (2001). Nonlinear developmental processes as sources of dominance. Genetics, 159(1), 423-432.
[5] Grafen, A. (1984) Natural selection, kin selection and group selection. In: Behavioural Ecology: An Evolutionary Approach,2nd edn (JR Krebs & NB Davies eds), pp. 62–84. Blackwell Scientific, Oxford.
[6] Grafen, A. (2006). A theory of Fisher's reproductive value. Journal of mathematical biology, 53(1), 15-60. doi: 10.1007/s00285-006-0376-4
[7] Hadfield, J. D., Nutall, A., Osorio, D. and Owens, I. P. F. (2007). Testing the phenotypic gambit: phenotypic, genetic and environmental correlations of colour. Journal of evolutionary biology, 20(2), 549-557. doi: 10.1111/j.1420-9101.2006.01262.x
[8] Kempes, C. P., West, G. B., and Koehl, M. (2019). The scales that limit: the physical boundaries of evolution. Frontiers in Ecology and Evolution, 7, 242. doi: 10.3389/fevo.2019.00242
[9] Kooijman, S. A. L. M. (2010) Dynamic Energy Budget theory for metabolic organisation. University Press, third edition.
[10] Metz, J. A. J., Mylius, S.D. and Diekman, O. (2008) When does evolution optimize?. Evolutionary Ecology Research 10: 629-654.
[11] Parker, G. A. (1974). Assessment strategy and the evolution of fighting behaviour. Journal of theoretical Biology, 47(1), 223-243. doi: 10.1016/0022-5193(74)90111-8
[12] Tonnabel, J., Van Dooren, T. J. M., Midgley, J., Haccou, P., Mignot, A., Ronce, O., and Olivieri, I. (2012). Optimal resource allocation in a serotinous non‐resprouting plant species under different fire regimes. Journal of Ecology, 100(6), 1464-1474. doi: 10.1111/j.1365-2745.2012.02023.x
[13] Van Dooren, T. J. M. (2000). The evolutionary dynamics of direct phenotypic overdominance: emergence possible, loss probable. Evolution, 54(6), 1899-1914. doi: 10.1111/j.0014-3820.2000.tb01236.x
[14] Van Dooren, T. J. M. (2006). Protected polymorphism and evolutionary stability in pleiotropic models with trait‐specific dominance. Evolution, 60(10), 1991-2003. doi: 10.1111/j.0014-3820.2006.tb01837.x
| Environmental perturbations and transitions between ecological and evolutionary equilibria: an eco-evolutionary feedback framework | Tim Coulson | <p>I provide a general framework for linking ecology and evolution. I start from the fact that individuals require energy, trace molecules, water, and mates to survive and reproduce, and that phenotypic resource accrual traits determine an individ... | 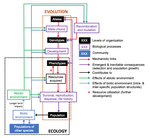 | Eco-evolutionary dynamics, Evolutionary ecology | Tom Van Dooren | | 2019-01-03 10:05:16 | View |
Which pitfall traps and sampling efforts should be used to evaluate the effects of cropping systems on the taxonomic and functional composition of arthropod communities?
Antoine Gardarin and Muriel Valantin-Morison
https://doi.org/10.5281/zenodo.3468920
On the importance of experimental design: pitfall traps and arthropod communities
Recommended by Ignasi Bartomeus based on reviews by Cécile ALBERT and Matthias Foellmer based on reviews by Cécile ALBERT and Matthias Foellmer
Despite the increasing refinement of statistical methods, a robust experimental design is still one of the most important cornerstones to answer ecological and evolutionary questions. However, there is a strong trade-off between a perfect design and its feasibility. A common mantra is that more data is always better, but how much is enough is complex to answer, specially when we want to capture the spatial and temporal variability of a given process. Gardarin and Valantin-Morison [1] make an effort to answer these questions for a practical case: How many pitfalls traps, of which type, and over which extent, do we need to detect shifts in arthropod community composition in agricultural landscapes. There is extense literature on how to approach these challenges using preliminary data in combination with simulation methods [e.g. 2], but practical cases are always welcomed to illustrate the complexity of the decisions to be made. A key challenge in this situation is the nature of simplified and patchy agricultural arthropod communities. In this context, small effect sizes are expected, but those small effects are relevant from an ecological point of view because small increases at low biodiversity may produce large gains in ecosystem functioning [3].
The paper shows that some variables are not important, such as the type of fluid used to fill the pitfall traps. This is good news for potential comparisons among studies using slightly different protocols. However, the bad news are that the sampling effort needed for detecting community changes is larger than the average effort currently implemented. A potential solution is to focus on Community Weighed Mean metrics (CWM; i.e. a functional descriptor of the community body size distribution) rather than on classic metrics such as species richness, as detecting changes on CWM requires a lower sampling effort and it has a clear ecological interpretation linked to ecosystem functioning.
Beyond the scope of the data presented, which is limited to a single region over two years, and hence it is hard to extrapolate to other regions and years, the big message of the paper is the need to incorporate statistical power simulations as a central piece of the ecologist's toolbox. This is challenging, especially when you face questions such as: Should I replicate over space, or over time? The recommended paper is accompanied by the statistical code used, which should facilitate this task to other researchers. Furthermore, we should be aware that some important questions in ecology are highly variable in space and time, and hence, larger sampling effort across space and time is needed to detect patterns. Larger and longer monitoring schemes require a large effort (and funding), but if we want to make relevant ecology, nobody said it would be easy.
References
[1] Gardarin, A. and Valantin-Morison, M. (2019). Which pitfall traps and sampling efforts should be used to evaluate the effects of cropping systems on the taxonomic and functional composition of arthropod communities? Zenodo, 3468920, ver. 3 peer-reviewed and recommended by PCI Ecology. doi: 10.5281/zenodo.3468920
[2] Johnson, P. C., Barry, S. J., Ferguson, H. M., and Müller, P. (2015). Power analysis for generalized linear mixed models in ecology and evolution. Methods in ecology and evolution, 6(2), 133-142. doi: 10.1111/2041-210X.12306
[3] Cardinale, B. J. et al. (2012). Biodiversity loss and its impact on humanity. Nature, 486(7401), 59-67. doi: 10.1038/nature11148
| Which pitfall traps and sampling efforts should be used to evaluate the effects of cropping systems on the taxonomic and functional composition of arthropod communities? | Antoine Gardarin and Muriel Valantin-Morison | <p>1. Ground dwelling arthropods are affected by agricultural practices, and analyses of their responses to different crop management are required. The sampling efficiency of pitfall traps has been widely studied in natural ecosystems. In arable a... |  | Agroecology, Biodiversity, Biological control, Community ecology | Ignasi Bartomeus | | 2019-01-08 09:40:14 | View |
Community size affects the signals of ecological drift and selection on biodiversity
Tadeu Siqueira, Victor S. Saito, Luis M. Bini, Adriano S. Melo, Danielle K. Petsch, Victor L. Landeiro, Kimmo T. Tolonen, Jenny Jyrkänkallio-Mikkola, Janne Soininen, Jani Heino
https://doi.org/10.1101/515098
Toward an empirical synthesis on the niche versus stochastic debate
Recommended by Eric Harvey based on reviews by Kevin Cazelles and Romain Bertrand
As far back as Clements [1] and Gleason [2], the historical schism between deterministic and stochastic perspectives has divided ecologists. Deterministic theories tend to emphasize niche-based processes such as environmental filtering and species interactions as the main drivers of species distribution in nature, while stochastic theories mainly focus on chance colonization, random extinctions and ecological drift [3]. Although the old days when ecologists were fighting fiercely over null models and their adequacy to capture niche-based processes is over [4], the ghost of that debate between deterministic and stochastic perspectives came back to haunt ecologists in the form of the ‘environment versus space’ debate with the development of metacommunity theory [5]. While interest in that question led to meaningful syntheses of metacommunity dynamics in natural systems [6], it also illustrated how context-dependant the answer was [7]. One of the next frontiers in metacommunity ecology is to identify the underlying drivers of this observed context-dependency in the relative importance of ecological processus [7, 8].
Reflecting on seminal work by Robert MacArthur emphasizing different processes at different spatial scales [9, 10] (the so-called ‘MacArthur paradox’), Chase and Myers proposed in 2011 that a key in solving the deterministic versus stochastic debate was probably to turn our attention to how the relative importance of local processes changes across spatial scales [3]. Scale-dependance is a well-acknowledged challenge in ecology, hampering empirical syntheses and comparisons between studies [11-14]. Embracing the scale-dependance of ecological processes would not only lead to stronger syntheses and consolidation of current knowledge, it could also help resolve many current debates or apparent contradictions [11, 15, 16].
The timely study by Siqueira et al. [17] fits well within this historical context by exploring the relative importance of ecological drift and selection across a gradient of community size (number of individuals in a given community). More specifically, they tested the hypothesis that small communities are more dissimilar among each other because of ecological drift compared to large communities, which are mainly structured by niche selection [17]. That smaller populations or communities should be more affected by drift is a mathematical given [18], but the main questions are i) for a given community size how important is ecological drift relative to other processes, and ii) how small does a community have to be before random assembly dominates? The authors answer these questions using an extensive stream dataset with a community size gradient sampled from 200 streams in two climatic regions (Brazil and Finland). Combining linear models with recent null model approaches to measure deviations from random expectations [19], they show that, as expected based on theory and recent experimental work, smaller communities tend to have higher β-diversity, and that those β-diversity patterns could not be distinguished from random assembly processes [17]. Spatial turnover among larger communities is mainly driven by niche-based processes related to species sorting or dispersal dynamics [17]. Given the current environmental context, with many anthropogenic perturbations leading to reduced community size, it is legitimate to wonder, as the authors do, whether we are moving toward a more stochastic and thus less predictable world with obvious implications for the conservation of biodiversity [17].
The real strength of the study by Siqueira et al. [17], in my opinion, is in the inclusion of stream data from boreal and tropical regions. Interestingly and most importantly, the largest communities in the tropical streams are as large as the smallest communities in the boreal streams. This is where the study should really have us reflect on the notions of context-dependency in observed patterns because the negative relationship between community size and β-diversity was only observed in the tropical streams, but not in the boreal streams [17]. This interesting nonlinearity in the response means that a study that would have investigated the drift versus niche-based question only in Finland would have found very different results from the same study in Brazil. Only by integrating such a large scale gradient of community sizes together could the authors show the actual shape of the relationship, which is the first step toward building a comprehensive synthesis on a debate that has challenged ecologists for almost a century.
References
[1] Clements, F. E. (1936). Nature and structure of the climax. Journal of ecology, 24(1), 252-284. doi: 10.2307/2256278
[2] Gleason, H. A. (1917). The structure and development of the plant association. Bulletin of the Torrey Botanical Club, 44(10), 463-481. doi: 10.2307/2479596
[3] Chase, J. M., and Myers, J. A. (2011). Disentangling the importance of ecological niches from stochastic processes across scales. Philosophical transactions of the Royal Society B: Biological sciences, 366(1576), 2351-2363. doi: 10.1098/rstb.2011.0063
[4] Diamond, J. M., and Gilpin, M. E. (1982). Examination of the “null” model of Connor and Simberloff for species co-occurrences on islands. Oecologia, 52(1), 64-74. doi: 10.1007/BF00349013
[5] Leibold M. A., et al. (2004). The metacommunity concept: a framework for multi‐scale community ecology. Ecology letters, 7(7), 601-613. doi: 10.1111/j.1461-0248.2004.00608.x
[6] Cottenie, K. (2005). Integrating environmental and spatial processes in ecological community dynamics. Ecology letters, 8(11), 1175-1182. doi: 10.1111/j.1461-0248.2005.00820.x
[7] Leibold, M. A. and Chase, J. M. (2018). Metacommunity Ecology. Monographs in Population Biology, vol. 59. Princeton University Press.
[8] Vellend, M. (2010). Conceptual synthesis in community ecology. The Quarterly review of biology, 85(2), 183-206. doi: 10.1086/652373
[9] MacArthur, R. H., and Wilson, E. O. (1963). An equilibrium theory of insular zoogeography. Evolution, 17(4), 373-387. doi: 10.1111/j.1558-5646.1963.tb03295.x
[10] MacArthur, R. H., and Levins, R. (1967). The limiting similarity, convergence, and divergence of coexisting species. The American Naturalist, 101(921), 377-385. doi: 10.1086/282505
[11] Viana, D. S., and Chase, J. M. (2019). Spatial scale modulates the inference of metacommunity assembly processes. Ecology, 100(2), e02576. doi: 10.1002/ecy.2576
[12] Chave, J. (2013). The problem of pattern and scale in ecology: what have we learned in 20 years?. Ecology letters, 16, 4-16. doi: 10.1111/ele.12048
[13] Patrick, C. J., and Yuan, L. L. (2019). The challenges that spatial context present for synthesizing community ecology across scales. Oikos, 128(3), 297-308. doi: 10.1111/oik.05802
[14] Chase, J. M., and Knight, T. M. (2013). Scale‐dependent effect sizes of ecological drivers on biodiversity: why standardised sampling is not enough. Ecology letters, 16, 17-26. doi: 10.1111/ele.12112
[15] Horváth, Z., Ptacnik, R., Vad, C. F., and Chase, J. M. (2019). Habitat loss over six decades accelerates regional and local biodiversity loss via changing landscape connectance. Ecology letters, 22(6), 1019-1027. doi: 10.1111/ele.13260
[16] Chase, J. M, Gooriah, L., May, F., Ryberg, W. A, Schuler, M. S, Craven, D., and Knight, T. M. (2019). A framework for disentangling ecological mechanisms underlying the island species–area relationship. Frontiers of Biogeography, 11(1). doi: 10.21425/F5FBG40844.
[17] Siqueira T., Saito V. S., Bini L. M., Melo A. S., Petsch D. K. , Landeiro V. L., Tolonen K. T., Jyrkänkallio-Mikkola J., Soininen J. and Heino J. (2019). Community size affects the signals of ecological drift and niche selection on biodiversity. bioRxiv 515098, ver. 4 peer-reviewed and recommended by PCI Ecology. doi: 10.1101/515098
[18] Hastings A., Gross L. J. eds. (2012). Encyclopedia of theoretical ecology (University of California Press, Berkeley).
[19] Chase, J. M., Kraft, N. J., Smith, K. G., Vellend, M., and Inouye, B. D. (2011). Using null models to disentangle variation in community dissimilarity from variation in α‐diversity. Ecosphere, 2(2), 1-11. doi: 10.1890/ES10-00117.1
| Community size affects the signals of ecological drift and selection on biodiversity | Tadeu Siqueira, Victor S. Saito, Luis M. Bini, Adriano S. Melo, Danielle K. Petsch, Victor L. Landeiro, Kimmo T. Tolonen, Jenny Jyrkänkallio-Mikkola, Janne Soininen, Jani Heino | <p>Ecological drift can override the effects of deterministic niche selection on small populations and drive the assembly of small communities. We tested the hypothesis that smaller local communities are more dissimilar among each other because of... |  | Biodiversity, Coexistence, Community ecology, Competition, Conservation biology, Dispersal & Migration, Freshwater ecology, Spatial ecology, Metacommunities & Metapopulations | Eric Harvey | | 2019-01-09 19:06:21 | View |
Does phenology explain plant-pollinator interactions at different latitudes? An assessment of its explanatory power in plant-hoverfly networks in French calcareous grasslands
Natasha de Manincor, Nina Hautekeete, Yves Piquot, Bertrand Schatz, Cédric Vanappelghem, François Massol
https://doi.org/10.5281/zenodo.2543768
The role of phenology for determining plant-pollinator interactions along a latitudinal gradient
Recommended by Anna Eklöf based on reviews by Ignasi Bartomeus, Phillip P.A. Staniczenko and 1 anonymous reviewer
Increased knowledge of what factors are determining species interactions are of major importance for our understanding of dynamics and functionality of ecological communities [1]. Currently, when ongoing temperature modifications lead to changes in species temporal and spatial limits the subject gets increasingly topical. A species phenology determines whether it thrive or survive in its environment. However, as the phenologies of different species are not necessarily equally affected by environmental changes, temporal or spatial mismatches can occur and affect the species-species interactions in the network [2] and as such the full network structure.
In this preprint by Manincor et al. [3] the authors explore the effect of phenology overlap on a large network of species interactions in calcareous grasslands in France. They analyze if and how this effect varies along a latitudinal gradient using empirical data on six plant-hoverfly networks. When comparing ecological network along gradients a well-known problem is that the network metrics is dependent on network size [4]. Therefore, instead of focusing on complete network structure the authors here focus on the factors that determine the probability of interactions and interaction frequency (number of visits). The authors use Bayesian Structural Equation Models (SEM) to link the interaction probability and number of visits to phenology overlap and species abundance. SEM is a multivariate technique that can be used to test several hypotheses and evaluate multiple causal relationships using both observed and latent variables to explain some other observed variables. The authors provide a nice description of the approach for this type of study system. In addition, the study also tests whether phenology affects network compartmentalization, by analyzing species subgroups using a latent block model (LBM) which is a clustering method particularly well-suited for weighted networks.
The authors identify phenology overlap as an important determinant of plant-pollinator interactions, but also conclude this factor alone is not sufficient to explain the species interactions. Species abundances was important for number of visits. Plant phenology drives the duration of the phenology overlap between plant and hoverflies in the studied system. This in turn influences either the probability of interaction or the expected number of visits, as well as network compartmentalization. Longer phenologies correspond to lower modularity inferring less constrained interactions, and shorter phenologies correspond to higher modularity inferring more constrained interactions.
What make this study particularly interesting is the presentation of SEMs as an innovative approach to compare networks of different sizes along environmental gradients. The authors show that these methods can be a useful tool when the aim is to understand the structure of plant-pollinator networks and data is varying in complexities. During the review process the authors carefully addressed to the comments from the two reviewers and the manuscript improved during the process. Both reviewers have expertise highly relevant for the research performed and the development of the manuscript. In my opinion this is a highly interesting and valuable piece of work both when it comes to the scientific question and the methodology. I look forward to further follow this research.
References
[1] Pascual, M., and Dunne, J. A. (Eds.). (2006). Ecological networks: linking structure to dynamics in food webs. Oxford University Press.
[2] Parmesan, C. (2007). Influences of species, latitudes and methodologies on estimates of phenological response to global warming. Global Change Biology, 13(9), 1860-1872. doi: 10.1111/j.1365-2486.2007.01404.x
[3] de Manincor, N., Hautekeete, N., Piquot, Y., Schatz, B., Vanappelghem, C. and Massol, F. (2019). Does phenology explain plant-pollinator interactions at different latitudes? An assessment of its explanatory power in plant-hoverfly networks in French calcareous grasslands. Zenodo, 2543768, ver. 4 peer-reviewed and recommended by PCI Ecology. doi: 10.5281/zenodo.2543768
[4] Staniczenko, P. P., Kopp, J. C., and Allesina, S. (2013). The ghost of nestedness in ecological networks. Nature communications, 4, 1391. doi: 10.1038/ncomms2422
| Does phenology explain plant-pollinator interactions at different latitudes? An assessment of its explanatory power in plant-hoverfly networks in French calcareous grasslands | Natasha de Manincor, Nina Hautekeete, Yves Piquot, Bertrand Schatz, Cédric Vanappelghem, François Massol | <p>For plant-pollinator interactions to occur, the flowering of plants and the flying period of pollinators (i.e. their phenologies) have to overlap. Yet, few models make use of this principle to predict interactions and fewer still are able to co... |  | Interaction networks, Pollination, Statistical ecology | Anna Eklöf | | 2019-01-18 19:02:13 | View |
A community perspective on the concept of marine holobionts: current status, challenges, and future directions
Simon M. Dittami, Enrique Arboleda, Jean-Christophe Auguet, Arite Bigalke, Enora Briand, Paco Cárdenas, Ulisse Cardini, Johan Decelle, Aschwin Engelen, Damien Eveillard, Claire M.M. Gachon, Sarah Griffiths, Tilmann Harder, Ehsan Kayal, Elena Kazamia, Francois H. Lallier, Mónica Medina, Ezequiel M. Marzinelli, Teresa Morganti, Laura Núñez Pons, Soizic Pardo, José Pintado Valverde, Mahasweta Saha, Marc-André Selosse, Derek Skillings, Willem Stock, Shinichi Sunagawa, Eve Toulza, Alexey Vorobev, Cat...
10.5281/zenodo.3696771
Marine holobiont in the high throughput sequencing era
Recommended by Sophie Arnaud-Haond and Corinne Vacher based on reviews by Sophie Arnaud-Haond and Aurélie Tasiemski
The concept of holobiont dates back to more than thirty years, it was primarily coined to hypothesize the importance of symbiotic associations to generate significant evolutionary novelties. Quickly adopted to describe the now well-studied system formed by zooxanthella associated corals, this concept expanded much further after the emergence of High-Throughput Sequencing and associated progresses in metabarcoding and metagenomics.
Holobionts – defined as the association between an individual host and its microbiota - are now increasingly described at sea and on land. The opinion article by Dittami et al. [1] provides a synthetic overview of marine holobionts. It retraces the history of the holobiont concept, recalls the main mechanisms underlying the association between hosts and microbial communities, highlights the influence of these symbioses on marine ecosystem functioning, and outlines current tools and future lines of research.
In particular, the article discusses some particularities of marine systems, such as the strong connectivity allowing an exchange of microorganisms and chemical signals between and within holobionts.
The authors advocate the need to bridge the gap between large scale exploration studies and smaller scale mechanistic studies, by conducting interdisciplinary research (combining physiology, biochemistry, ecology, experimentation and computational modeling) on some keystone holobionts.
Finally, one strength of the paper by Dittami et al. [1] is that it places the concept of the holobiont in an applied research framework. Several possible applications of knowledge on host-microbiota interactions are suggested, both in the field of aquaculture and that of monitoring the health of marine ecosystems. This article contains all the necessary elements for someone who would like to jump into the study of the holobionths in the marine world.
References
[1] Dittami SM, Arboleda E, Auguet J, Bigalke A, Briand E, Cardenas P, Cardini U, Decelle J, Engelen AH, Eveillard D, Gachon CMM, Griffiths SM, Harder T, Kayal E, Kazamia E, Lallier FH, Medina M, Marzinelli E, Morganti T, Núñez Pons L, Prado S, Pintado J, Saha M, Selosse M, Skillings D, Stock W, Sunagawa S, Toulza E, Vorobev A, Leblanc C, Not F. (2020). A community perspective on the concept of marine holobionts: current status, challenges, and future directions. Zenodo, ver. 4 peer-reviewed and recommended by PCI Ecology. doi: 10.5281/zenodo.3696771
| A community perspective on the concept of marine holobionts: current status, challenges, and future directions | Simon M. Dittami, Enrique Arboleda, Jean-Christophe Auguet, Arite Bigalke, Enora Briand, Paco Cárdenas, Ulisse Cardini, Johan Decelle, Aschwin Engelen, Damien Eveillard, Claire M.M. Gachon, Sarah Griffiths, Tilmann Harder, Ehsan Kayal, Elena Kazam... | Host-microbe interactions play crucial roles in marine ecosystems. However, we still have very little understanding of the mechanisms that govern these relationships, the evolutionary processes that shape them, and their ecological consequences. T... |  | Marine ecology, Microbial ecology & microbiology, Symbiosis | Sophie Arnaud-Haond | | 2019-02-05 17:57:11 | View |
Interplay between historical and current features of the cityscape in shaping the genetic structure of the house mouse (Mus musculus domesticus) in Dakar (Senegal, West Africa)
Claire Stragier, Sylvain Piry, Anne Loiseau, Mamadou Kane, Aliou Sow, Youssoupha Niang, Mamoudou Diallo, Arame Ndiaye, Philippe Gauthier, Marion Borderon, Laurent Granjon, Carine Brouat, Karine Berthier
https://doi.org/10.1101/557066
Urban past predicts contemporary genetic structure in city rats
Recommended by Michelle DiLeo based on reviews by Torsti Schulz, ? and 1 anonymous reviewer
Urban areas are expanding worldwide, and have become a dominant part of the landscape for many species. Urbanization can fragment pre-existing populations of vulnerable species leading to population declines and the loss of connectivity. On the other hand, expansion of urban areas can also facilitate the spread of human commensals including pests. Knowledge of the features of cityscapes that facilitate gene flow and maintain diversity of pests is thus key to their management and eradication.
Cities are complex mosaics of natural and manmade surfaces, and habitat quality is not only influenced by physical aspects of the cityscape but also by socioeconomic factors and human behaviour. Constant development means that cities also change rapidly in time; contemporary urban life reflects only a snapshot of the environmental conditions faced by populations. It thus remains a challenge to identify the features that actually drive ecology and evolution of populations in cities [1]. While several studies have highlighted strong urban clines in genetic structure and adaption [2], few have considered the influence of factors beyond physical aspects of the cityscape or historical processes.
In this paper, Stragier et al. [3] sought to identify the current and past features of the cityscape and socioeconomic factors that shape genetic structure and diversity of the house mouse (Mus musculus domesticus) in Dakar, Senegal. The authors painstakingly digitized historical maps of Dakar from the time of European settlement in 1862 to present. The authors found that the main spatial genetic cline was best explained by historical cityscape features, with higher apparent gene flow and genetic diversity in areas that were connected earlier to initial European settlements. Beyond the main trend of spatial genetic structure, they found further evidence that current features of the cityscape were important. Specifically, areas with low vegetation and poor housing conditions were found to support large, genetically diverse populations. The authors demonstrate that their results are reproducible using several statistical approaches, including modeling that explicitly accounts for spatial autocorrelation.
The work of Stragier et al. [3] thus highlights that populations of city-dwelling species are the product of both past and present cityscapes. Going forward, urban evolutionary ecologists should consider that despite the potential for rapid evolution in urban landscapes, the signal of a species’ colonization can remain for generations.
References
[1] Rivkin, L. R., Santangelo, J. S., Alberti, M. et al. (2019). A roadmap for urban evolutionary ecology. Evolutionary Applications, 12(3), 384-398. doi: 10.1111/eva.12734
[2] Miles, L. S., Rivkin, L. R., Johnson, M. T., Munshi‐South, J. and Verrelli, B. C. (2019). Gene flow and genetic drift in urban environments. Molecular ecology, 28(18), 4138-4151. doi: 10.1111/mec.15221
[3] Stragier, C., Piry, S., Loiseau, A., Kane, M., Sow, A., Niang, Y., Diallo, M., Ndiaye, A., Gauthier, P., Borderon, M., Granjon, L., Brouat, C. and Berthier, K. (2020). Interplay between historical and current features of the cityscape in shaping the genetic structure of the house mouse (Mus musculus domesticus) in Dakar (Senegal, West Africa). bioRxiv, 557066, ver. 4 peer-reviewed and recommended by PCI Ecology. doi: 10.1101/557066
| Interplay between historical and current features of the cityscape in shaping the genetic structure of the house mouse (Mus musculus domesticus) in Dakar (Senegal, West Africa) | Claire Stragier, Sylvain Piry, Anne Loiseau, Mamadou Kane, Aliou Sow, Youssoupha Niang, Mamoudou Diallo, Arame Ndiaye, Philippe Gauthier, Marion Borderon, Laurent Granjon, Carine Brouat, Karine Berthier | <p>Population genetic approaches may be used to investigate dispersal patterns of species living in highly urbanized environment in order to improve management strategies for biodiversity conservation or pest control. However, in such environment,... |  | Biological invasions, Landscape ecology, Molecular ecology | Michelle DiLeo | | 2019-02-22 08:36:13 | View |
Niche complementarity among pollinators increases community-level plant reproductive success
Ainhoa Magrach, Francisco P. Molina, Ignasi Bartomeus
https://doi.org/10.1101/629931
Improving our knowledge of species interaction networks
Recommended by Cédric Gaucherel based on reviews by Michael Lattorff, Nicolas Deguines and 3 anonymous reviewers based on reviews by Michael Lattorff, Nicolas Deguines and 3 anonymous reviewers
Ecosystems shelter a huge number of species, continuously interacting. Each species interact in various ways, with trophic interactions, but also non-trophic interactions, not mentioning the abiotic and anthropogenic interactions. In particular, pollination, competition, facilitation, parasitism and many other interaction types are simultaneously present at the same place in terrestrial ecosystems [1-2]. For this reason, we need today to improve our understanding of such complex interaction networks to later anticipate their responses. This program is a huge challenge facing ecologists and they today join their forces among experimentalists, theoreticians and modelers. While some of us struggle in theoretical and modeling dimensions [3-4], some others perform brilliant works to observe and/or experiment on the same ecological objects [5-6].
In this nice study [6], Magrach et al. succeed in studying relatively large plant-pollinator interaction networks in the field, in Mediterranean ecosystems. For the first time to my knowledge, they study community-wide interactions instead of traditional and easier accessible pairwise interactions. On the basis of a statistically relevant survey, they focus on plant reproductive success and on the role of pollinator interactions in such a success. A more reductionist approach based on simpler pairwise interactions between plants and pollinators would not be able to highlight the interaction network structure (the topology) possibly impacting its responses [1,5], among which the reproductive success of some (plant) species. Yet, such a network analysis requires a fine control of probable biases, as those linked to size or autocorrelation between data of various sites. Here, Magrach et al. did a nice work in capturing rigorously the structures and trends behind this community-wide functioning.
To grasp possible relationships between plant and pollinator species is a first mandatory step, but the next critical step requires understanding processes hidden behind such relationships. Here, the authors succeed to reach this step too, by starting interpreting the processes at stake in their studied plant-pollinator networks [7]. In particular, the niche complementarity has been demonstrated to play a determinant role in the plant reproductive success, and has a positive impact on it [6].
When will we be able to detect a community-wise process? This is one of my team’s objectives, and we developed new kind of models with this aim. Also, authors focus here on plant-pollinator network, but the next step might be to gather every kind of interactions into a huge ecosystem network which we call the socio-ecosystemic graph [4]. Indeed, why to limit our view to certain interactions only? It will take time to grasp the whole interaction network an ecosystem is sheltering, but this should be our next challenge. And this paper of Magrach et al. [6] is a first fascinating step in this direction.
References
[1] Campbell, C., Yang, S., Albert, R., and Shea, K. (2011). A network model for plant–pollinator community assembly. Proceedings of the National Academy of Sciences, 108(1), 197-202. doi: 10.1073/pnas.1008204108
[2] Kéfi, S., Miele, V., Wieters, E. A., Navarrete, S. A., and Berlow, E. L. (2016). How structured is the entangled bank? The surprisingly simple organization of multiplex ecological networks leads to increased persistence and resilience. PLoS biology, 14(8), e1002527. doi: 10.1371/journal.pbio.1002527
[3] Gaucherel, C. (2019). The Languages of Nature. When nature writes to itself. Lulu editions, Paris, France.
[4] Gaucherel, C., and Pommereau, F. Using discrete systems to exhaustively characterize the dynamics of an integrated ecosystem. Methods in Ecology and Evolution, 10(9), 1615-1627. doi: 10.1111/2041-210X.13242
[5] Bennett, J. M. et al. (2018). A review of European studies on pollination networks and pollen limitation, and a case study designed to fill in a gap. AoB Plants, 10(6), ply068. doi: 10.1093/aobpla/ply068
[6] Magrach, A., Molina, F. P., and Bartomeus, I. (2020). Niche complementarity among pollinators increases community-level plant reproductive success. bioRxiv, 629931, ver. 7 peer-reviewed and recommended by PCI Ecology. doi: 10.1101/629931
[7] Bastolla, U., Fortuna, M. A., Pascual-García, A., Ferrera, A., Luque, B., and Bascompte, J. (2009). The architecture of mutualistic networks minimizes competition and increases biodiversity. Nature, 458(7241), 1018-1020. doi: 10.1038/nature07950
| Niche complementarity among pollinators increases community-level plant reproductive success | Ainhoa Magrach, Francisco P. Molina, Ignasi Bartomeus | <p>Declines in pollinator diversity and abundance have been reported across different regions, with implications for the reproductive success of plant species. However, research has focused primarily on pairwise plant-pollinator interactions, larg... |  | Ecosystem functioning, Interaction networks, Pollination, Terrestrial ecology | Cédric Gaucherel | Nicolas Deguines | 2019-05-07 17:03:23 | View |



 based on reviews by Joaquín Hortal and 2 anonymous reviewers
based on reviews by Joaquín Hortal and 2 anonymous reviewers








 based on reviews by Cécile ALBERT and Matthias Foellmer
based on reviews by Cécile ALBERT and Matthias Foellmer








 based on reviews by Michael Lattorff, Nicolas Deguines and 3 anonymous reviewers
based on reviews by Michael Lattorff, Nicolas Deguines and 3 anonymous reviewers











