Direct submissions to PCI Ecology from bioRxiv.org are possible using the B2J service
Latest recommendations

| Id | Title * ▲ | Authors * | Abstract * | Picture * | Thematic fields * | Recommender | Reviewers | Submission date | |
|---|---|---|---|---|---|---|---|---|---|
06 May 2021

Trophic niche of the invasive gregarious species Crepidula fornicata, in relation to ontogenic changesThibault Androuin, Stanislas F. Dubois, Cédric Hubas, Gwendoline Lefebvre, Fabienne Le Grand, Gauthier Schaal, Antoine Carlier https://doi.org/10.1101/2020.07.30.229021A lack of clear dietary differences between ontogenetic stages of invasive slippersnails provides important insights into resource use and potential inter- and intra-specific competitionRecommended by Matthew Bracken based on reviews by 2 anonymous reviewersThe slippersnail (Crepidula fornicata), originally from the eastern coast of North America, has invaded European coastlines from Norway to the Mediterranean Sea [1]. This species is capable of achieving incredibly high densities (up to several thousand individuals per square meter) and likely has major impacts on a variety of community- and ecosystem-level processes, including alteration of carbon and nitrogen fluxes and competition with native suspension feeders [2]. Given this potential for competition, it is important to understand the diet of C. fornicata and its potential overlap with native species. However, previous research on the diet of C. fornicata and related species suggests that the types of food consumed may change with age [3, 4]. This species has an unusual reproductive strategy. It is a sequential hermaphrodite, which begins life as a somewhat mobile male but eventually slows down to become sessile. Sessile individuals form stacks of up to 10 or more individuals, with larger individuals on the bottom of the stack, and decreasingly smaller individuals piled on top. Snails at the bottom of the stack are female, whereas snails at the top of the stack are male; when the females die, the largest males become female [5]. Thus, understanding these potential ontogenetic dietary shifts has implications for both intraspecific (juvenile vs. male vs. female) and interspecific competition associated with an abundant, invasive species. To this end, Androuin and colleagues evaluated the stable-isotope (d13C and d15N) and fatty-acid profiles of food sources and different life-history stages of C. fornicata [6]. Based on previous work highlighting the potential for life-history changes in the diet of this species [3,4], they hypothesized that C. fornicata would shift its diet as it aged and predicted that this shift would be reflected in changes in its stable-isotope and fatty-acid profiles. The authors found that potential food sources (biofilm, suspended particulate organic matter, and superficial sedimentary organic matter) differed substantially in both stable-isotope and fatty-acid signatures. However, whereas fatty-acid profiles changed substantially with age, there was no shift in the stable-isotope signatures. Because stable-isotope differences between food sources were not reflected in differences between life-history stages, the authors conservatively concluded that there was insufficient evidence for a diet shift with age. The ontogenetic shifts in fatty-acid profiles were intriguing, but the authors suggested that these reflected age-related physiological changes rather than changes in diet. The authors’ work highlights the need to consider potential changes in the roles of invasive species with age, especially when evaluating interactions with native species. In this case, C. fornicata consumed a variety of food sources, including both benthic and particulate organic matter, regardless of age. The carbon stable-isotope signature of C. fornicata overlaps with those of several native suspension- and deposit-feeding species in the region [7], suggesting the possibility of resource competition, especially given the high abundances of this invader. This contribution demonstrates the potential difficulty of characterizing the impacts of an abundant invasive species with a complex life-history strategy. Like many invasive species, C. fornicata appears to be a dietary generalist, which likely contributes to its success in establishing and thriving in a variety of locations [8].
References [1] Blanchard M (1997) Spread of the slipper limpet Crepidula fornicata (L. 1758) in Europe. Current state dans consequences. Scientia Marina, 61, 109–118. Open Access version : https://archimer.ifremer.fr/doc/00423/53398/54271.pdf [2] Martin S, Thouzeau G, Chauvaud L, Jean F, Guérin L, Clavier J (2006) Respiration, calcification, and excretion of the invasive slipper limpet, Crepidula fornicata L.: Implications for carbon, carbonate, and nitrogen fluxes in affected areas. Limnology and Oceanography, 51, 1996–2007. https://doi.org/10.4319/lo.2006.51.5.1996 [3] Navarro JM, Chaparro OR (2002) Grazing–filtration as feeding mechanisms in motile specimens of Crepidula fecunda (Gastropoda: Calyptraeidae). Journal of Experimental Marine Biology and Ecology, 270, 111–122. https://doi.org/10.1016/S0022-0981(02)00013-8 [4] Yee AK, Padilla DK (2015) Allometric Scaling of the Radula in the Atlantic Slippersnail Crepidula fornicata. Journal of Shellfish Research, 34, 903–907. https://doi.org/10.2983/035.034.0320 [5] Collin R (1995) Sex, Size, and Position: A Test of Models Predicting Size at Sex Change in the Protandrous Gastropod Crepidula fornicata. The American Naturalist, 146, 815–831. https://doi.org/10.1086/285826 [6] Androuin T, Dubois SF, Hubas C, Lefebvre G, Grand FL, Schaal G, Carlier A (2021) Trophic niche of the invasive gregarious species Crepidula fornicata, in relation to ontogenic changes. bioRxiv, 2020.07.30.229021, ver. 4 peer-reviewed and recommended by Peer Community in Ecology. https://doi.org/10.1101/2020.07.30.229021 [7] Dauby P, Khomsi A, Bouquegneau J-M (1998) Trophic Relationships within Intertidal Communities of the Brittany Coasts: A Stable Carbon Isotope Analysis. Journal of Coastal Research, 14, 1202–1212. Retrieved May 4, 2021, from http://www.jstor.org/stable/4298880 [8] Machovsky-Capuska GE, Senior AM, Simpson SJ, Raubenheimer D (2016) The Multidimensional Nutritional Niche. Trends in Ecology & Evolution, 31, 355–365. https://doi.org/10.1016/j.tree.2016.02.009
| Trophic niche of the invasive gregarious species Crepidula fornicata, in relation to ontogenic changes | Thibault Androuin, Stanislas F. Dubois, Cédric Hubas, Gwendoline Lefebvre, Fabienne Le Grand, Gauthier Schaal, Antoine Carlier | <p style="text-align: justify;">The slipper limpet Crepidula fornicata is a common and widespread invasive gregarious species along the European coast. Among its life-history traits, well-documented ontogenic changes in behavior (i.e., motile male... |  | Food webs, Life history, Marine ecology | Matthew Bracken | 2020-08-01 23:55:57 | View | |
23 Jan 2024

Use of linear features by red-legged partridges in an intensive agricultural landscape: implications for landscape management in farmlandCharlotte Perrot, Antoine Berceaux, Mathias Noel, Beatriz Arroyo, Leo Bacon https://doi.org/10.1101/2023.07.27.550774The importance of managing linear features in agricultural landscapes for farmland birdsRecommended by Ricardo Correia based on reviews by Matthew Grainger and 1 anonymous reviewerEuropean farmland bird populations continue declining at an alarming rate, and some species require urgent action to avoid their demise (Silva et al. 2024). While factors such as climate change and urbanization also play an important role in driving the decline of farmland bird populations, its main driver seems to be linked with agricultural intensification (Rigal et al. 2023). Besides increased pesticide and fertilizer use, agricultural intensification often results in the homogenization of agricultural landscapes through the removal of seminatural linear features such as hedgerows, field margins, and grassy strips that can be beneficial for biodiversity. These features may be particularly important during the breeding season, when breeding farmland birds can benefit from patches of denser vegetation to conceal nests and improve breeding success. It is both important and timely to understand how landscape management can help to address the ongoing decline of farmland birds by identifying specific actions that can boost breeding success. Perrot et al. 2023 contribute to this effort by exploring how red-legged partridges use linear features in an intensive agricultural landscape during the breeding season. Through a combination of targeted fieldwork and GPS tracking, the authors highlight patterns in home range size and habitat selection that provide insights for landscape management. Specifically, their results suggest that birds have smaller range sizes in the vicinity of traffic routes and seminatural features structured by both herbaceous and woody cover. Furthermore, they show that breeding birds tend to choose linear elements with herbaceous cover whereas non-breeders prefer linear elements with woody cover, underlining the importance of accounting for the needs of both breeding and non-breeding birds. In particular, the authors stress the importance of providing additional vegetation elements such as hedges, grassy strips or embankments in order to increase landscape heterogeneity. These landscape elements are usually found in the vicinity of linear infrastructures such as roads and tracks, but it is important they are available also in separate areas to avoid the risk of bird collision and the authors provide specific recommendations towards this end. Overall, this is an important study with clear recommendations on how to improve landscape management for these farmland birds. References Perrot, C., Séranne, L., Berceaux, A., Noel, M., Arroyo, B., & Bacon, L. (2023) "Use of linear features by red-legged partridges in an intensive agricultural landscape: implications for landscape management in farmland." bioRxiv, ver. 2 peer-reviewed and recommended by Peer Community in Ecology. | Use of linear features by red-legged partridges in an intensive agricultural landscape: implications for landscape management in farmland | Charlotte Perrot, Antoine Berceaux, Mathias Noel, Beatriz Arroyo, Leo Bacon | <p>Current agricultural practices and change are the major cause of biodiversity loss. An important change associated with the intensification of agriculture in the last 50 years is the spatial homogenization of the landscape with substantial loss... |  | Agroecology, Behaviour & Ethology, Biodiversity, Conservation biology, Habitat selection | Ricardo Correia | 2023-08-01 10:27:33 | View | |
05 Apr 2019

Using a large-scale biodiversity monitoring dataset to test the effectiveness of protected areas at conserving North-American breeding birdsVictor Cazalis, Soumaya Belghali, Ana S.L. Rodrigues https://doi.org/10.1101/433037Protected Areas effects on biodiversity: a test using bird data that hopefully will give ideas for much more studies to comeRecommended by Paul Caplat based on reviews by Willson Gaul and 1 anonymous reviewerIn the face of worldwide declines in biodiversity, evaluating the effectiveness of conservation practices is an absolute necessity. Protected Areas (PA) are a key tool for conservation, and the question “Are PA effective” has been on many a research agenda, as the introduction to this preprint will no doubt convince you. A challenge we face is that, until now, few studies have been explicitly designed to evaluate PA, and despite the rise of meta-analyses on the topic, our capacity to quantify their effect on biodiversity remains limited. References [1] Cazalis, V., Belghali, S., & Rodrigues, A. S. (2019). Using a large-scale biodiversity monitoring dataset to test the effectiveness of protected areas at conserving North-American breeding birds. bioRxiv, 433037, ver. 4 peer-reviewed and recommended by PCI Ecology. doi: 10.1101/433037 | Using a large-scale biodiversity monitoring dataset to test the effectiveness of protected areas at conserving North-American breeding birds | Victor Cazalis, Soumaya Belghali, Ana S.L. Rodrigues | <p>Protected areas currently cover about 15% of the global land area, and constitute one of the main tools in biodiversity conservation. Quantifying their effectiveness at protecting species from local decline or extinction involves comparing prot... |  | Biodiversity, Conservation biology, Human impact, Landscape ecology, Macroecology | Paul Caplat | 2018-10-04 08:43:34 | View | |
29 May 2023

Using integrated multispecies occupancy models to map co-occurrence between bottlenose dolphins and fisheries in the Gulf of Lion, French Mediterranean SeaValentin Lauret, Hélène Labach, Léa David, Matthieu Authier, Olivier Gimenez https://doi.org/10.32942/osf.io/npd6uMapping co-occurence of human activities and wildlife from multiple data sourcesRecommended by Paul Caplat based on reviews by Mason Fidino and 1 anonymous reviewerTwo fields of research have grown considerably over the past twenty years: the investigation of human-wildlife conflicts (e.g. see Treves & Santiago-Ávila 2020), and multispecies occupancy modelling (Devarajan et al. 2020). In their recent study, Lauret et al. (2023) combined both in an elegant methodological framework, applied to the study of the co-occurrence of fishing activities and bottlenose dolphins in the French Mediterranean. A common issue with human-wildlife conflicts (and, in particular, fishery by-catch) is that data is often only available from those conflicts or interactions, limiting the validity of the predictions (Kuiper et al. 2022). Lauret et al. use independent data sources informing the occurrence of fishing vessels and dolphins, combined in a Bayesian multispecies occupancy model where vessels are "the other species". I particularly enjoyed that approach, as integration of human activities in ecological models can be extremely complex, but can also translate in phenomena that can be captured as one would of individuals of a species, as long as the assumptions are made clearly. Here, the model is made more interesting by accounting for environmental factors (seabed depth) borrowing an approach from Generalized Additive Models in the Bayesian framework. While not pretending to provide (yet) practical recommendations to help conserve bottlenose dolphins (and other wildlife conflicts), this study and the associated code are a promising step in that direction. REFERENCES Devarajan, K., Morelli, T.L. & Tenan, S. (2020), Multi-species occupancy models: review, roadmap, and recommendations. Ecography, 43: 1612-1624. https://doi.org/10.1111/ecog.04957 Kuiper, T., Loveridge, A.J. and Macdonald, D.W. (2022), Robust mapping of human–wildlife conflict: controlling for livestock distribution in carnivore depredation models. Anim. Conserv., 25: 195-207. https://doi.org/10.1111/acv.12730 Lauret V, Labach H, David L, Authier M, & Gimenez O (2023) Using integrated multispecies occupancy models to map co-occurrence between bottlenose dolphins and fisheries in the Gulf of Lion, French Mediterranean Sea. Ecoevoarxiv, ver. 2 peer-reviewed and recommended by PCI Ecology. https://doi.org/10.32942/osf.io/npd6u Treves, A. & Santiago-Ávila, F.J. (2020). Myths and assumptions about human-wildlife conflict and coexistence. Conserv. Biol. 34, 811–818. https://doi.org/10.1111/cobi.13472 | Using integrated multispecies occupancy models to map co-occurrence between bottlenose dolphins and fisheries in the Gulf of Lion, French Mediterranean Sea | Valentin Lauret, Hélène Labach, Léa David, Matthieu Authier, Olivier Gimenez | <p style="text-align: justify;">In the Mediterranean Sea, interactions between marine species and human activities are prevalent. The coastal distribution of bottlenose dolphins (<em>Tursiops truncatus</em>) and the predation pressure they put on ... |  | Marine ecology, Population ecology, Species distributions | Paul Caplat | 2022-10-21 11:13:36 | View | |
26 May 2023
Using repeatability of performance within and across contexts to validate measures of behavioral flexibilityMcCune KB, Blaisdell AP, Johnson-Ulrich Z, Lukas D, MacPherson M, Seitz BM, Sevchik A, Logan CJ https://doi.org/10.32942/X2R59KDo reversal learning methods measure behavioral flexibility?Recommended by Aurélie Coulon based on reviews by Maxime Dahirel and Aparajitha Ramesh based on reviews by Maxime Dahirel and Aparajitha Ramesh
Assessing the reliability of the methods we use in actually measuring the intended trait should be one of our first priorities when designing a study – especially when the trait in question is not directly observable and is measured through a proxy. This is the case for cognitive traits, which are often quantified through measures of behavioral performance. Behavioral flexibility is of particular interest in the context of great environmental changes that a lot of populations have to experiment. This type of behavioral performance is often measured through reversal learning experiments (Bond 2007). In these experiments, individuals first learn a preference, for example for an object of a certain type of form or color, associated with a reward such as food. The characteristics of the rewarded object then change, and the individuals hence have to learn these new characteristics (to get the reward). The time needed by the individual to make this change in preference has been considered a measure of behavioral flexibility. Although reversal learning experiments have been widely used, their construct validity to assess behavioral flexibility has not been thoroughly tested. This was the aim of McCune and collaborators' (2023) study, through the test of the repeatability of individual performance within and across contexts of reversal learning, in the great-tailed grackle. This manuscript presents a post-study of the preregistered study* (Logan et al. 2019) that was peer-reviewed and received an In Principle Recommendation for PCI Ecology (Coulon 2019; the initial preregistration was split into 3 post-studies).
The first hypothesis was tested by measuring the repeatability of the time needed by individuals to switch color preference in a color reversal learning task (colored tubes), over serial sessions of this task. The second one was tested by measuring the time needed by individuals to switch solutions, within 3 different contexts: (1) colored tubes, (2) plastic and (3) wooden multi-access boxes involving several ways to access food. Despite limited sample sizes, the results of these experiments suggest that there is both temporal and contextual repeatability of behavioral flexibility performance of great-tailed grackles, as measured by reversal learning experiments. Those results are a first indication of the construct validity of reversal learning experiments to assess behavioral flexibility. As highlighted by McCune and collaborators, it is now necessary to assess the discriminant validity of these experiments, i.e. checking that a different performance is obtained with tasks (experiments) that are supposed to measure different cognitive abilities. Coulon, A. (2019) Can context changes improve behavioral flexibility? Towards a better understanding of species adaptability to environmental changes. Peer Community in Ecology, 100019. https://doi.org/10.24072/pci.ecology.100019 Logan, CJ, Lukas D, Bergeron L, Folsom M, & McCune, K. (2019). Is behavioral flexibility related to foraging and social behavior in a rapidly expanding species? In Principle Acceptance by PCI Ecology of the Version on 6 Aug 2019. http://corinalogan.com/Preregistrations/g_flexmanip.html McCune KB, Blaisdell AP, Johnson-Ulrich Z, Lukas D, MacPherson M, Seitz BM, Sevchik A, Logan CJ (2023) Using repeatability of performance within and across contexts to validate measures of behavioral flexibility. EcoEvoRxiv, ver. 5 peer-reviewed and recommended by Peer Community in Ecology. https://doi.org/10.32942/X2R59K | Using repeatability of performance within and across contexts to validate measures of behavioral flexibility | McCune KB, Blaisdell AP, Johnson-Ulrich Z, Lukas D, MacPherson M, Seitz BM, Sevchik A, Logan CJ | <p style="text-align: justify;">Research into animal cognitive abilities is increasing quickly and often uses methods where behavioral performance on a task is assumed to represent variation in the underlying cognitive trait. However, because thes... | Behaviour & Ethology, Evolutionary ecology, Preregistrations, Zoology | Aurélie Coulon | 2022-08-15 20:56:42 | View | ||
18 Dec 2019
Validating morphological condition indices and their relationship with reproductive success in great-tailed gracklesJennifer M. Berens, Corina J. Logan, Melissa Folsom, Luisa Bergeron, Kelsey B. McCune https://github.com/corinalogan/grackles/blob/master/Files/Preregistrations/gcondition.RmdAre condition indices positively related to each other and to fitness?: a test with gracklesRecommended by Marcos Mendez based on reviews by Javier Seoane and Isabel López-RullReproductive succes, as a surrogate of individual fitness, depends both on extrinsic and intrinsic factors [1]. Among the intrinsic factors, resource level or health are considered important potential drivers of fitness but exceedingly difficult to measure directly. Thus, a host of proxies have been suggested, known as condition indices [2]. The question arises whether all condition indices consistently measure the same "inner state" of individuals and whether all of them similarly correlate to individual fitness. In this preregistration, Berens and colleagues aim to answer this question for two common condition indices, fat score and scaled mass index (Fig. 1), using great-tailed grackles as a model system. Although this question is not new, it has not been satisfactorily solved and both reviewers found merit in the attempt to clarify this matter. References [1] Roff, D. A. (2001). Life history evolution. Oxford University Press, Oxford. | Validating morphological condition indices and their relationship with reproductive success in great-tailed grackles | Jennifer M. Berens, Corina J. Logan, Melissa Folsom, Luisa Bergeron, Kelsey B. McCune | Morphological variation among individuals has the potential to influence multiple life history characteristics such as dispersal, migration, reproductive fitness, and survival (Wilder, Raubenheimer, and Simpson (2016)). Theoretically, individuals ... | Behaviour & Ethology, Conservation biology, Demography, Morphometrics, Preregistrations, Zoology | Marcos Mendez | 2019-08-05 20:05:56 | View | ||
20 Sep 2018
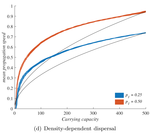
When higher carrying capacities lead to faster propagationMarjorie Haond, Thibaut Morel-Journel, Eric Lombaert, Elodie Vercken, Ludovic Mailleret & Lionel Roques https://doi.org/10.1101/307322When the dispersal of the many outruns the dispersal of the fewRecommended by Matthieu Barbier based on reviews by Yuval Zelnik and 1 anonymous reviewer based on reviews by Yuval Zelnik and 1 anonymous reviewer
Are biological invasions driven by a few pioneers, running ahead of their conspecifics? Or are these pioneers constantly being caught up by, and folded into, the larger flux of propagules from the established populations behind them? References [1] Levins, R., & Culver, D. (1971). Regional Coexistence of Species and Competition between Rare Species. Proceedings of the National Academy of Sciences, 68(6), 1246–1248. doi: 10.1073/pnas.68.6.1246 | When higher carrying capacities lead to faster propagation | Marjorie Haond, Thibaut Morel-Journel, Eric Lombaert, Elodie Vercken, Ludovic Mailleret & Lionel Roques | <p>This preprint has been reviewed and recommended by Peer Community In Ecology (https://dx.doi.org/10.24072/pci.ecology.100004). Finding general patterns in the expansion of natural populations is a major challenge in ecology and invasion biology... |  | Biological invasions, Colonization, Dispersal & Migration, Experimental ecology, Population ecology, Spatial ecology, Metacommunities & Metapopulations, Theoretical ecology | Matthieu Barbier | Yuval Zelnik | 2018-04-25 10:18:48 | View |
07 Oct 2019

Which pitfall traps and sampling efforts should be used to evaluate the effects of cropping systems on the taxonomic and functional composition of arthropod communities?Antoine Gardarin and Muriel Valantin-Morison https://doi.org/10.5281/zenodo.3468920On the importance of experimental design: pitfall traps and arthropod communitiesRecommended by Ignasi Bartomeus based on reviews by Cécile ALBERT and Matthias Foellmer based on reviews by Cécile ALBERT and Matthias Foellmer
Despite the increasing refinement of statistical methods, a robust experimental design is still one of the most important cornerstones to answer ecological and evolutionary questions. However, there is a strong trade-off between a perfect design and its feasibility. A common mantra is that more data is always better, but how much is enough is complex to answer, specially when we want to capture the spatial and temporal variability of a given process. Gardarin and Valantin-Morison [1] make an effort to answer these questions for a practical case: How many pitfalls traps, of which type, and over which extent, do we need to detect shifts in arthropod community composition in agricultural landscapes. There is extense literature on how to approach these challenges using preliminary data in combination with simulation methods [e.g. 2], but practical cases are always welcomed to illustrate the complexity of the decisions to be made. A key challenge in this situation is the nature of simplified and patchy agricultural arthropod communities. In this context, small effect sizes are expected, but those small effects are relevant from an ecological point of view because small increases at low biodiversity may produce large gains in ecosystem functioning [3]. References [1] Gardarin, A. and Valantin-Morison, M. (2019). Which pitfall traps and sampling efforts should be used to evaluate the effects of cropping systems on the taxonomic and functional composition of arthropod communities? Zenodo, 3468920, ver. 3 peer-reviewed and recommended by PCI Ecology. doi: 10.5281/zenodo.3468920 | Which pitfall traps and sampling efforts should be used to evaluate the effects of cropping systems on the taxonomic and functional composition of arthropod communities? | Antoine Gardarin and Muriel Valantin-Morison | <p>1. Ground dwelling arthropods are affected by agricultural practices, and analyses of their responses to different crop management are required. The sampling efficiency of pitfall traps has been widely studied in natural ecosystems. In arable a... |  | Agroecology, Biodiversity, Biological control, Community ecology | Ignasi Bartomeus | 2019-01-08 09:40:14 | View | |
21 Oct 2020

Why scaling up uncertain predictions to higher levels of organisation will underestimate changeJames Orr, Jeremy Piggott, Andrew Jackson, Jean-François Arnoldi https://doi.org/10.1101/2020.05.26.117200Uncertain predictions of species responses to perturbations lead to underestimate changes at ecosystem level in diverse systemsRecommended by Elisa Thebault based on reviews by Carlos Melian and 1 anonymous reviewerDifferent sources of uncertainty are known to affect our ability to predict ecological dynamics (Petchey et al. 2015). However, the consequences of uncertainty on prediction biases have been less investigated, especially when predictions are scaled up to higher levels of organisation as is commonly done in ecology for instance. The study of Orr et al. (2020) addresses this issue. It shows that, in complex systems, the uncertainty of unbiased predictions at a lower level of organisation (e.g. species level) leads to a bias towards underestimation of change at higher level of organisation (e.g. ecosystem level). This bias is strengthened by larger uncertainty and by higher dimensionality of the system. References Cardinale BJ, Duffy JE, Gonzalez A, Hooper DU, Perrings C, Venail P, Narwani A, Mace GM, Tilman D, Wardle DA, Kinzig AP, Daily GC, Loreau M, Grace JB, Larigauderie A, Srivastava DS, Naeem S (2012) Biodiversity loss and its impact on humanity. Nature, 486, 59–67. https://doi.org/10.1038/nature11148 | Why scaling up uncertain predictions to higher levels of organisation will underestimate change | James Orr, Jeremy Piggott, Andrew Jackson, Jean-François Arnoldi | <p>Uncertainty is an irreducible part of predictive science, causing us to over- or underestimate the magnitude of change that a system of interest will face. In a reductionist approach, we may use predictions at the level of individual system com... |  | Community ecology, Ecosystem functioning, Theoretical ecology | Elisa Thebault | Anonymous | 2020-06-02 15:41:12 | View |
MANAGING BOARD
Julia Astegiano
Tim Coulson
Anna Eklof
Dominique Gravel
François Massol
Ben Phillips
Cyrille Violle











