Direct submissions to PCI Ecology from bioRxiv.org are possible using the B2J service
Latest recommendations

| Id | Title * | Authors * | Abstract * | Picture * | Thematic fields * | Recommender▲ | Reviewers | Submission date | |
|---|---|---|---|---|---|---|---|---|---|
21 Nov 2023
Pathogen community composition and co-infection patterns in a wild community of rodentsJessica Lee Abbate, Maxime Galan, Maria Razzauti, Tarja Sironen, Liina Voutilainen, Heikki Henttonen, Patrick Gasqui, Jean-François Cosson, Nathalie Charbonnel https://doi.org/10.1101/2020.02.09.940494Reservoirs of pestilence: what pathogen and rodent community analyses can tell us about transmission riskRecommended by Francois Massol based on reviews by Adrian Diaz, Romain Pigeault and 1 anonymous reviewer based on reviews by Adrian Diaz, Romain Pigeault and 1 anonymous reviewer
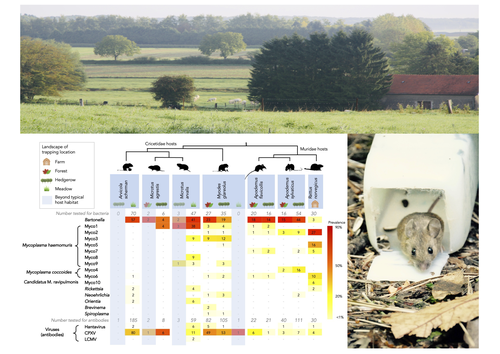
Rodents are well known as one of the main animal groups responsible for human-transmitted pathogens. As such, it seems logical to try and survey what kinds of pathogenic microbes might be harboured by wild rodents, in order to establish some baseline surveillance and prevent future zoonotic outbreaks (Bernstein et al., 2022). This is exactly what Abbate et al. (2023) endeavoured and their findings are intimidating. Based on quite a large sampling effort, they collected more than 700 rodents of seven species around two villages in northeastern France. They looked for molecular markers indicative of viral and bacterial infections and proceeded to analyze their pathogen communities using multivariate techniques. Variation in the prevalence of the different pathogens was found among host species, with e.g. signs of CPXV more prevalent in Cricetidae while some Mycoplasma strains were more prevalent in Muridae. Co-circulation of pathogens was found in all species, with some evidencing signs of up to 12 different pathogen taxa. The diversity of co-circulating pathogens was markedly different between host species and higher in adult hosts, but not affected by sex. The dataset also evinced some slight differences between habitats, with meadows harbouring a little more diversity of rodent pathogens than forests. Less intuitively, some pathogen associations seemed quite repeatable, such as the positive association of Bartonella spp. with CPXV in the montane water vole. The study allowed the authors to test several associations already described in the literature, including associations between different hemotropic Mycoplasma species. I strongly invite colleagues interested in zoonoses, emerging pandemics and more generally One Health to read the paper of Abbate et al. (2023) and try to replicate them across the world. To prevent the next sanitary crises, monitoring rodents, and more generally vertebrates, population demographics is a necessary and enlightening step (Johnson et al., 2020), but insufficient. Following the lead of colleagues working on rodent ectoparasites (Krasnov et al., 2014), we need more surveys like the one described by Abbate et al. (2023) to understand the importance of the dilution effect in the prevalence and transmission of microbial pathogens (Andreazzi et al., 2023) and the formation of epidemics. We also need other similar studies to assess the potential of different rodent species to carry pathogens more or less capable of infecting other mammalian species (Morand et al., 2015), in other places in the world. References Abbate, J. L., Galan, M., Razzauti, M., Sironen, T., Voutilainen, L., Henttonen, H., Gasqui, P., Cosson, J.-F. & Charbonnel, N. (2023) Pathogen community composition and co-infection patterns in a wild community of rodents. BioRxiv, ver.4 peer-reviewed and recommended by Peer Community in Ecology. https://doi.org/10.1101/2020.02.09.940494 Andreazzi, C. S., Martinez-Vaquero, L. A., Winck, G. R., Cardoso, T. S., Teixeira, B. R., Xavier, S. C. C., Gentile, R., Jansen, A. M. & D'Andrea, P. S. (2023) Vegetation cover and biodiversity reduce parasite infection in wild hosts across ecological levels and scales. Ecography, 2023, e06579. | Pathogen community composition and co-infection patterns in a wild community of rodents | Jessica Lee Abbate, Maxime Galan, Maria Razzauti, Tarja Sironen, Liina Voutilainen, Heikki Henttonen, Patrick Gasqui, Jean-François Cosson, Nathalie Charbonnel | <p style="text-align: justify;">Rodents are major reservoirs of pathogens that can cause disease in humans and livestock. It is therefore important to know what pathogens naturally circulate in rodent populations, and to understand the factors tha... |  | Biodiversity, Coexistence, Community ecology, Eco-immunology & Immunity, Epidemiology, Host-parasite interactions, Population ecology, Species distributions | Francois Massol | 2020-02-11 12:42:28 | View | |
19 Feb 2020

Soil variation response is mediated by growth trajectories rather than functional traits in a widespread pioneer Neotropical treeSébastien Levionnois, Niklas Tysklind, Eric Nicolini, Bruno Ferry, Valérie Troispoux, Gilles Le Moguedec, Hélène Morel, Clément Stahl, Sabrina Coste, Henri Caron, Patrick Heuret https://doi.org/10.1101/351197Growth trajectories, better than organ-level functional traits, reveal intraspecific response to environmental variationRecommended by François Munoz based on reviews by Georges Kunstler and François Munoz based on reviews by Georges Kunstler and François Munoz
Functional traits are “morpho-physio-phenological traits which impact fitness indirectly via their effects on growth, reproduction and survival” [1]. Most functional traits are defined at organ level, e.g. for leaves, roots and stems, and reflect key aspects of resource acquisition and resource use by organisms for their development and reproduction [2]. More rarely, some functional traits can be related to spatial development, such as vegetative height and lateral spread in plants. References [1] Violle, C., Navas, M. L., Vile, D., Kazakou, E., Fortunel, C., Hummel, I., & Garnier, E. (2007). Let the concept of trait be functional!. Oikos, 116(5), 882-892. doi: 10.1111/j.0030-1299.2007.15559.x | Soil variation response is mediated by growth trajectories rather than functional traits in a widespread pioneer Neotropical tree | Sébastien Levionnois, Niklas Tysklind, Eric Nicolini, Bruno Ferry, Valérie Troispoux, Gilles Le Moguedec, Hélène Morel, Clément Stahl, Sabrina Coste, Henri Caron, Patrick Heuret | <p style="text-align: justify;">1- Trait-environment relationships have been described at the community level across tree species. However, whether interspecific trait-environment relationships are consistent at the intraspecific level is yet unkn... |  | Botany, Eco-evolutionary dynamics, Habitat selection, Ontogeny, Tropical ecology | François Munoz | 2018-06-21 17:13:17 | View | |
22 Nov 2021
When more competitors means less harvested resourceRecommended by François Munoz based on reviews by Francois Massol, Jeremy Van Cleve and 1 anonymous reviewer based on reviews by Francois Massol, Jeremy Van Cleve and 1 anonymous reviewer
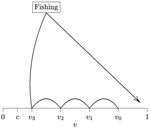
In this paper, Alan R. Rogers (2021) examines the dynamics of foraging strategies for a resource that gains value over time (e.g., ripening fruits), while there is a fixed cost of attempting to forage the resource, and once the resource is harvested nothing is left for other harvesters. For this model, not any pure foraging strategy is evolutionary stable. A mixed equilibrium exists, i.e., with a mixture of foraging strategies within the population, which is still evolutionarily unstable. Nonetheless, Alan R. Rogers shows that for a large number of competitors and/or high harvesting cost, the mixture of strategies remains close to the mixed equilibrium when simulating the dynamics. Surprisingly, in a large population individuals will less often attempt to forage the resource and will instead “go fishing”. The paper also exposes an experiment of the game with students, which resulted in a strategy distribution somehow close to the theoretical mixture of strategies. The economist John F. Nash Jr. (1950) gained the Nobel Prize of economy in 1994 for his game theoretical contributions. He gave his name to the “Nash equilibrium”, which represents a set of individual strategies that is reached whenever all the players have nothing to gain by changing their strategy while the strategies of others are unchanged. Alan R. Rogers shows that the mixed equilibrium in the foraging game is such a Nash equilibrium. Yet it is evolutionarily unstable insofar as a distribution close to the equilibrium can invade. The insights of the study are twofold. First, it sheds light on the significance of Nash equilibrium in an ecological context of foraging strategies. Second, it shows that an evolutionarily unstable state can rule the composition of the ecological system. Therefore, the contribution made by the paper should be most significant to better understand the dynamics of competitive communities and their eco-evolutionary trajectories. References Nash JF (1950) Equilibrium points in n-person games. Proceedings of the National Academy of Sciences, 36, 48–49. https://doi.org/10.1073/pnas.36.1.48 Rogers AR (2021) Beating your Neighbor to the Berry Patch. bioRxiv, 2020.11.12.380311, ver. 8 peer-reviewed and recommended by Peer Community in Ecology. https://doi.org/10.1101/2020.11.12.380311
| Beating your neighbor to the berry patch | Alan R. Rogers | <p style="text-align: justify;">Foragers often compete for resources that ripen (or otherwise improve) gradually. What strategy is optimal in this situation? It turns out that there is no optimal strategy. There is no evolutionarily stable strateg... |  | Behaviour & Ethology, Evolutionary ecology, Foraging | François Munoz | Erol Akçay, Jorge Peña, Sébastien Lion, François Rousset, Ulf Dieckmann , Troy Day , Corina Tarnita , Florence Debarre , Daniel Friedman , Vlastimil Krivan , Ulf Dieckmann | 2020-12-10 18:38:49 | View |
01 Mar 2022

Dissimilarity of species interaction networks: quantifying the effect of turnover and rewiringTimothée Poisot https://doi.org/10.32942/osf.io/gxhu2How to evaluate and interpret the contribution of species turnover and interaction rewiring when comparing ecological networks?Recommended by François Munoz based on reviews by Ignasi Bartomeus and 1 anonymous reviewer based on reviews by Ignasi Bartomeus and 1 anonymous reviewer
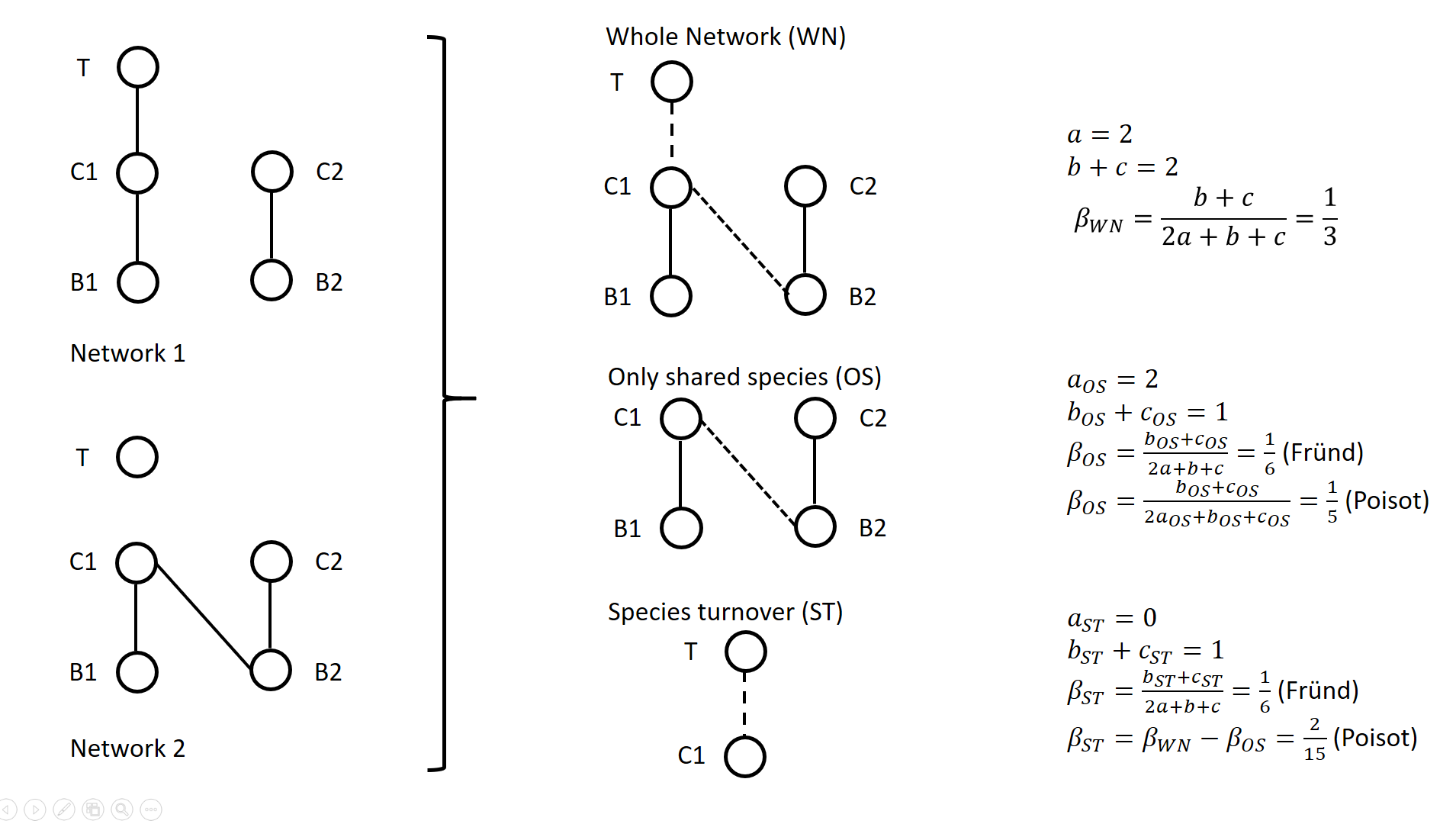
A network includes a set of vertices or nodes (e.g., species in an interaction network), and a set of edges or links (e.g., interactions between species). Whether and how networks vary in space and/or time are questions often addressed in ecological research. Two ecological networks can differ in several extents: in that species are different in the two networks and establish new interactions (species turnover), or in that species that are present in both networks establish different interactions in the two networks (rewiring). The ecological meaning of changes in network structure is quite different according to whether species turnover or interaction rewiring plays a greater role. Therefore, much attention has been devoted in recent years on quantifying and interpreting the relative changes in network structure due to species turnover and/or rewiring. Poisot et al. (2012) proposed to partition the global variation in structure between networks, \( \beta_{WN} \) (WN = Whole Network) into two terms: \( \beta_{OS} \) (OS = Only Shared species) and \( \beta_{ST} \) (ST = Species Turnover), such as \( \beta_{WN} = \beta_{OS} + \beta_{ST} \). The calculation lays on enumerating the interactions between species that are common or not to two networks, as illustrated on Figure 1 for a simple case. Specifically, Poisot et al. (2012) proposed to use a Sorensen type measure of network dissimilarity, i.e., \( \beta_{WN} = \frac{a+b+c}{(2a+b+c)/2} -1=\frac{b+c}{2a+b+c} \) , where \( a \) is the number of interactions shared between the networks, while \( b \) and \( c \) are interaction numbers unique to one and the other network, respectively. \( \beta_{OS} \) is calculated based on the same formula, but only for the subnetworks including the species common to the two networks, in the form \( \beta_{OS} = \frac{b_{OS}+c_{OS}}{2a_{OS}+b_{OS}+c_{OS}} \) (e.g., Fig. 1). \( \beta_{ST} \) is deduced by subtracting \( \beta_{OS} \) from \( \beta_{WN} \) and represents in essence a "dissimilarity in interaction structure introduced by dissimilarity in species composition" (Poisot et al. 2012).
Figure 1. Ecological networks exemplified in Fründ (2021) and discussed in Poisot (2022). a is the number of shared links (continuous lines in right figures), while b+c is the number of edges unique to one or the other network (dashed lines in right figures). Alternatively, Fründ (2021) proposed to define \( \beta_{OS} = \frac{b_{OS}+c_{OS}}{2a+b+c} \) and \( \beta_{ST} = \frac{b_{ST}+c_{ST}}{2a+b+c} \), where \( b_{ST}=b-b_{OS} \) and \( c_{ST}=c-c_{OS} \) , so that the components \( \beta_{OS} \) and \( \beta_{ST} \) have the same denominator. In this way, Fründ (2021) partitioned the count of unique \( b+c=b_{OS}+b_{ST}+c_{ST} \) interactions, so that \( \beta_{OS} \) and \( \beta_{ST} \) sums to \( \frac{b_{OS}+c_{OS}+b_{ST}+c_{ST}}{2a+b+c} = \frac{b+c}{2a+b+c} = \beta_{WN} \). Fründ (2021) advocated that this partition allows a more sensible comparison of \( \beta_{OS} \) and \( \beta_{ST} \), in terms of the number of links that contribute to each component. For instance, let us consider the networks 1 and 2 in Figure 1 (left panel) such as \( a_{OS}=2 \) (continuous lines in right panel), \( b_{ST} + c_{ST} = 1 \) and \( b_{OS} + c_{OS} = 1 \) (dashed lines in right panel), and thereby \( a = 2 \), \( b+c=2 \), \( \beta_{WN} = 1/3 \). Fründ (2021) measured \( \beta_{OS}=\beta_{ST}=1/6 \) and argued that it is appropriate insofar as it reflects that the number of unique links in the OS and ST components contributing to network dissimilarity (dashed lines) are actually equal. Conversely, the formula of Poisot et al. (2012) yields \( \beta_{OS}=1/5 \), hence \( \beta_{ST} = \frac{1}{3}-\frac{1}{5}=\frac{2}{15}<\beta_{OS} \). Fründ (2021) thus argued that the method of Poisot tends to underestimate the contribution of species turnover. To clarify and avoid misinterpretation of the calculation of \( \beta_{OS} \) and \( \beta_{ST} \) in Poisot et al. (2012), Poisot (2022) provides a new, in-depth mathematical analysis of the decomposition of \( \beta_{WN} \). Poisot et al. (2012) quantify in \( \beta_{OS} \) the actual contribution of rewiring in network structure for the subweb of common species. Poisot (2022) thus argues that \( \beta_{OS} \) relates only to the probability of rewiring in the subweb, while the definition of \( \beta_{OS} \) by Fründ (2021) is relative to the count of interactions in the global network (considered in denominator), and is thereby dependent on both rewiring probability and species turnover. Poisot (2022) further clarifies the interpretation of \( \beta_{ST} \). \( \beta_{ST} \) is obtained by subtracting \( \beta_{OS} \) from \( \beta_{WN} \) and thus represents the influence of species turnover in terms of the relative architectures of the global networks and of the subwebs of shared species. Coming back to the example of Fig.1., the Poisot et al. (2012) formula posits that \( \frac{\beta_{ST}}{\beta_{WN}}=\frac{2/15}{1/3}=2/5 \), meaning that species turnover contributes two-fifths of change in network structure, while rewiring in the subweb of common species contributed three fifths. Conversely, the approach of Fründ (2021) does not compare the architectures of global networks and of the subwebs of shared species, but considers the relative contribution of unique links to network dissimilarity in terms of species turnover and rewiring. Poisot (2022) concludes that the partition proposed in Fründ (2021) does not allow unambiguous ecological interpretation of rewiring. He provides guidelines for proper interpretation of the decomposition proposed in Poisot et al. (2012). References Fründ J (2021) Dissimilarity of species interaction networks: how to partition rewiring and species turnover components. Ecosphere, 12, e03653. https://doi.org/10.1002/ecs2.3653 Poisot T, Canard E, Mouillot D, Mouquet N, Gravel D (2012) The dissimilarity of species interaction networks. Ecology Letters, 15, 1353–1361. https://doi.org/10.1111/ele.12002 Poisot T (2022) Dissimilarity of species interaction networks: quantifying the effect of turnover and rewiring. EcoEvoRxiv Preprints, ver. 4 peer-reviewed and recommended by Peer Community in Ecology. https://doi.org/10.32942/osf.io/gxhu2 | Dissimilarity of species interaction networks: quantifying the effect of turnover and rewiring | Timothée Poisot | <p style="text-align: justify;">Despite having established its usefulness in the last ten years, the decomposition of ecological networks in components allowing to measure their β-diversity retains some methodological ambiguities. Notably, how to ... |  | Biodiversity, Interaction networks, Theoretical ecology | François Munoz | 2021-07-31 00:18:41 | View | |
07 Jun 2023
High intraspecific growth variability despite strong evolutionary heritage in a neotropical forestSylvain Schmitt, Bruno Hérault, Géraldine Derroire https://doi.org/10.1101/2022.07.27.501745Environmental and functional determinants of tree performance in a neotropical forest: the imprint of evolutionary legacy on growth strategiesRecommended by François Munoz based on reviews by David Murray-Stoker, Camille Girard and Jelena Pantel based on reviews by David Murray-Stoker, Camille Girard and Jelena Pantel
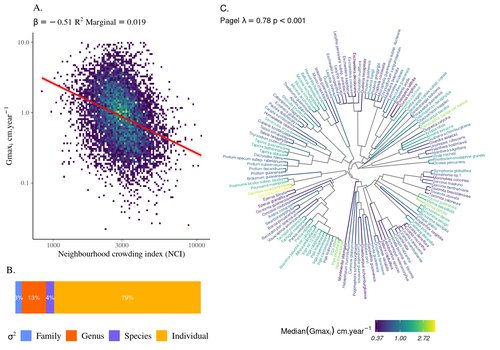
The hyperdiverse tropical forests have long fascinated ecologists because the fact that so many species persist at a low density at a local scale remains hard to explain. Both niche-based and neutral hypotheses have been tested, primarily based on analyzing the taxonomic composition of tropical forest plots (Janzen 1970; Hubbell 2001). Studies of the functional and phylogenetic structure of tropical tree communities have further aimed to better assess the importance of niche-based processes. For instance, Baraloto et al. (2012) found that co-occurring species were functionally and phylogenetically more similar in a neotropical forest, suggesting a role of environmental filtering. Likewise, Schmitt et al. (2021) found the influence of environmental filtering on the functional composition of an Indian rainforest. Yet these studies evidenced non-random trait-environment association based on the composition of assemblages only (in terms of occurrences and abundances). A major challenge remains to further address whether and how tree performance varies among species and individuals in tropical forests. Functional traits are related to components of individual fitness (Violle et al. 2007). Recently, more and more emphasis has been put on examining the relationship between functional trait values and demographic parameters (Salguero-Gómez et al. 2018), in order to better understand how functional trait values determine species population dynamics and abundances in assemblages. Fortunel et al. (2018) found an influence of functional traits on species growth variation related to topography, and less clearly to neighborhood density (crowding). Poorter et al. (2018) observed 44% of trait variation within species in a neotropical forest. Although individual trait values would be expected to be better predictors of performance than average values measured at the species level, Poorter et al still found a poor relationship. Schmitt et al. (2023) examined how abiotic conditions and biotic interactions (considering neighborhood density) influenced the variation of individual potential tree growth, in a tropical forest plot located in French Guiana. They also considered the link between species-averaged values of growth potential and functional traits. Schmitt et al. (2023) found substantial variation in growth potential within species, that functional traits explained 40% of the variation of species-averaged growth and, noticeably, that the taxonomic structure (used as random effect in their model) explained a third of the variation in individual growth. Although functional traits of roots, wood and leaves could predict a significant part of species growth potential, much variability of tree growth occurred within species. Intraspecific trait variation can thus be huge in response to changing abiotic and biotic contexts across individuals. The information on phylogenetic relationships can still provide a proxy of the integrated phenotypic variation that is under selection across the phylogeny, and determine a variation in growth strategies among individuals. The similarity of the phylogenetic structure suggests a joint selection of these growth strategies and related functional traits during events of convergent evolution. Baraloto et al. (2012) already noted that phylogenetic distance can be a proxy of niche overlap in tropical tree communities. Here, Schmitt et al. further demonstrate that evolutionary heritage is significantly related to individual growth variation, and plead for better acknowledging this role in future studies. While the role of fitness differences in tropical tree community dynamics remained to be assessed, the present study provides new evidence that individual growth does vary depending on evolutionary relationships, which can reflect the roles of selection and adaptation on growth strategies. Therefore, investigating both the influence of functional traits and phylogenetic relationships on individual performance remains a promising avenue of research, for functional and community ecology in general. REFERENCES Baraloto, Christopher, Olivier J. Hardy, C. E. Timothy Paine, Kyle G. Dexter, Corinne Cruaud, Luke T. Dunning, Mailyn-Adriana Gonzalez, et al. 2012. « Using functional traits and phylogenetic trees to examine the assembly of tropical tree communities ». Journal of Ecology, 100: 690‑701. | High intraspecific growth variability despite strong evolutionary heritage in a neotropical forest | Sylvain Schmitt, Bruno Hérault, Géraldine Derroire | <p style="text-align: justify;">Individual tree growth is a key determinant of species performance and a driver of forest dynamics and composition. Previous studies on tree growth unravelled the variation in species growth as a function of demogra... |  | Community ecology, Demography, Population ecology | François Munoz | Jelena Pantel, David Murray-Stoker | 2022-08-01 14:29:04 | View |
13 May 2024
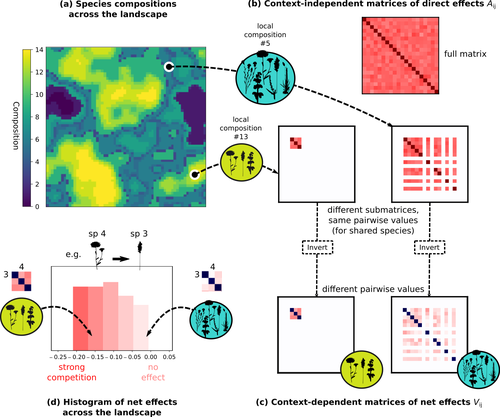
Getting More by Asking for Less: Linking Species Interactions to Species Co-Distributions in MetacommunitiesMatthieu Barbier, Guy Bunin, Mathew A. Leibold https://doi.org/10.1101/2023.06.04.543606Beyond pairwise species interactions: coarser inference of their joined effects is more relevantRecommended by François Munoz based on reviews by Frederik De Laender, Hao Ran Lai and Malyon Bimler based on reviews by Frederik De Laender, Hao Ran Lai and Malyon Bimler
Barbier et al. (2024) investigated the dynamics of species abundances depending on their ecological niche (abiotic component) and on (numerous) competitive interactions. In line with previous evidence and expectations (Barbier et al. 2018), the authors show that it is possible to robustly infer the mean and variance of interaction coefficients from species co-distributions, while it is not possible to infer the individual coefficient values. The authors devised a simulation framework representing multispecies dynamics in an heterogeneous environmental context (2D grid landscape). They used a Lotka-Volterra framework involving pairwise interaction coefficients and species-specific carrying capacities. These capacities depend on how well the species niche matches the local environmental conditions, through a Gaussian function of the distance of the species niche centers to the local environmental values. They considered two contrasted scenarios denoted as « Environmental tracking » and « Dispersal limited ». In the latter case, species are initially seeded over the environmental grid and cannot disperse to other cells, while in the former case they can disperse and possibly be more performant in other cells. The direct effects of species on one another are encoded in an interaction matrix A, and the authors further considered net interactions depending on the inverse of the matrix of direct interactions (Zelnik et al., 2024). The net effects are context-dependent, i.e., it involves the environment-dependent biotic capacities, even through the interaction terms can be defined between species as independent from local environment. The results presented here underline that the outcome of many individual competitive interactions can only be understood in terms of macroscopic properties. In essence, the results here echoe the mean field theories that investigate the dynamics of average ecological properties instead of the microscopic components (e.g., McKane et al. 2000). In a philosophical perspective, community ecology has long struggled with analyzing and inferring local determinants of species coexistence from species co-occurrence patterns, so that it was claimed that no universal laws can be derived in the discipline (Lawton 1999). Using different and complementary methods and perspectives, recent research has also shown that species assembly parameter values cannot be unambiguously inferred from species co-occurrences only, even in simple designs where an equilibrium can be reached (Poggiato et al. 2021). Although the roles of high-order competitive interactions and intransivity can lead to species coexistence, the simple view of a single loop of competitive interactions is easily challenged when further interactions and complexity is added (Gallien et al. 2024). But should we put so much emphasis on inferring individual interaction coefficients? In a quest to understand the emerging properties of elementary processes, ecological theory could go forward with a more macroscopic analysis and understanding of species coexistence in many communities. The authors referred several times to an interesting paper from Schaffer (1981), entitled « Ecological abstraction: the consequences of reduced dimensionality in ecological models ». It proposes that estimating individual species competition coefficients is not possible, but that competition can be assessed at the coarser level of organisation, i.e., between ecological guilds. This idea implies that the dimensionality of the competition equations should be greatly reduced to become tractable in practice. Taking together this claim with the results of the present Barbier et al. (2024) paper, it becomes clearer that the nature of competitive interactions can be addressed through « abstracted » quantities, as those of guilds or the moments of the individual competition coefficients (here the average and the standard deviation). Therefore the scope of Barbier et al. (2024) framework goes beyond statistical issues in parameter inference, but question the way we must think and represent the numerous competitive interactions in a simplified and robust way. References Barbier, Matthieu, Jean-François Arnoldi, Guy Bunin, et Michel Loreau. 2018. « Generic assembly patterns in complex ecological communities ». Proceedings of the National Academy of Sciences 115 (9): 2156‑61. https://doi.org/10.1073/pnas.1710352115 | Getting More by Asking for Less: Linking Species Interactions to Species Co-Distributions in Metacommunities | Matthieu Barbier, Guy Bunin, Mathew A. Leibold | <p>AbstractOne of the more difficult challenges in community ecology is inferring species interactions on the basis of patterns in the spatial distribution of organisms. At its core, the problem is that distributional patterns reflect the ‘realize... |  | Biogeography, Community ecology, Competition, Spatial ecology, Metacommunities & Metapopulations, Species distributions, Statistical ecology, Theoretical ecology | François Munoz | 2023-10-21 14:14:16 | View | |
02 Oct 2018
How optimal foragers should respond to habitat changes? On the consequences of habitat conversion.Vincent Calcagno, Frederic Hamelin, Ludovic Mailleret, Frederic Grognard 10.1101/273557Optimal foraging in a changing world: old questions, new perspectivesRecommended by Francois-Xavier Dechaume-Moncharmont based on reviews by Frederick Adler, Andrew Higginson and 1 anonymous reviewer based on reviews by Frederick Adler, Andrew Higginson and 1 anonymous reviewer
Marginal value theorem (MVT) is an archetypal model discussed in every behavioural ecology textbook. Its popularity is largely explained but the fact that it is possible to solve it graphically (at least in its simplest form) with the minimal amount of equations, which is a sensible strategy for an introductory course in behavioural ecology [1]. Apart from this heuristic value, one may be tempted to disregard it as a naive toy model. After a burst of interest in the 70's and the 80's, the once vivid literature about optimal foraging theory (OFT) has lost its momentum [2]. Yet, OFT and MVT have remained an active field of research in the parasitoidologists community, mostly because the sampling strategy of a parasitoid in patches of hosts and its resulting fitness gain are straightforward to evaluate, which eases both experimental and theoretical investigations [3]. References [1] Fawcett, T. W. & Higginson, A. D. 2012 Heavy use of equations impedes communication among biologists. Proc. Natl. Acad. Sci. 109, 11735–11739. doi: 10.1073/pnas.1205259109 | How optimal foragers should respond to habitat changes? On the consequences of habitat conversion. | Vincent Calcagno, Frederic Hamelin, Ludovic Mailleret, Frederic Grognard | The Marginal Value Theorem (MVT) provides a framework to predict how habitat modifications related to the distribution of resources over patches should impact the realized fitness of individuals and their optimal rate of movement (or patch residen... |  | Behaviour & Ethology, Dispersal & Migration, Foraging, Landscape ecology, Spatial ecology, Metacommunities & Metapopulations, Theoretical ecology | Francois-Xavier Dechaume-Moncharmont | 2018-03-05 10:42:11 | View | |
01 Feb 2020

Touchy matter: the delicate balance between Morgan’s canon and open-minded description of advanced cognitive skills in the animalRecommended by Francois-Xavier Dechaume-Moncharmont based on reviews by Valérie Dufour and Alex Taylor based on reviews by Valérie Dufour and Alex Taylor
In a recent paper published in PNAS, Fayet et al. [1] reported scarce field observations of two Atlantic puffins (four years apart) apparently scratching their bodies using sticks, which was interpreted by the authors as evidence of tool use in this species. In a short response, Benjamin Farrar [2] raises serious concerns about this interpretation and proposes simpler, more parsimonious, mechanisms explaining the observed behaviour: a textbook case of Morgan's canon. References [1] Fayet, A. L., Hansen, E. S., and Biro, D. (2020). Evidence of tool use in a seabird. Proceedings of the National Academy of Sciences, 117(3), 1277–1279. doi: 10.1073/pnas.1918060117 | Evidence of tool use in a seabird? | Benjamin G. Farrar | Fayet, Hansen and Biro (1) provide two observations of Atlantic puffins, *Fratercula arctica*, performing self-directed actions while holding a stick in their beaks. The authors interpret this as evidence of tool use as they suggest that the stick... |  | Behaviour & Ethology | Francois-Xavier Dechaume-Moncharmont | 2020-01-22 11:55:27 | View | |
28 Sep 2020
The dynamics of spawning acts by a semelparous fish and its associated energetic costsCédric Tentelier, Colin Bouchard, Anaïs Bernardin, Amandine Tauzin, Jean-Christophe Aymes, Frédéric Lange, Charlotte Recapet, Jacques Rives https://doi.org/10.1101/436295Extreme weight loss: when accelerometer could reveal reproductive investment in a semelparous fish speciesRecommended by Francois-Xavier Dechaume-Moncharmont based on reviews by Aidan Jonathan Mark Hewison, Loïc Teulier and 1 anonymous reviewer based on reviews by Aidan Jonathan Mark Hewison, Loïc Teulier and 1 anonymous reviewer
Continuous observation of animal behaviour could be quite a challenge in the field, and the situation becomes even more complicated with aquatic species mostly active at night. In such cases, biologging techniques are real game changers in ecology, behavioural ecology or eco-physiology. An accelerating number of methodological applications of these tools in natural condition are thus published each year [1]. Biologging is not limited to movement ecology. For instance, fine grain information about energy expenditure can be inferred from body acceleration [2], and accelerometers has already proven useful in monitoring reproductive costs in some fish species [3,4]. The first part of the study by Tentelier et al. [5] is in line with this growing literature. It describes measurements of energy expenditure during reproduction in a fish species, Allis shad (Alosa Alosa), based on tail beat frequency and occurrence of spawning acts. The study has been convincingly conducted, and the results are important for fish biologists. But this is not the whole story: the authors added to this otherwise classical study a very original and insightful analysis which deserves closer interest. References [1] Börger L, Bijleveld AI, Fayet AL, Machovsky‐Capuska GE, Patrick SC, Street GM and Vander Wal E. (2020) Biologging special feature. J. Anim. Ecol. 89, 6–15. 10.1111/1365-2656.13163 | The dynamics of spawning acts by a semelparous fish and its associated energetic costs | Cédric Tentelier, Colin Bouchard, Anaïs Bernardin, Amandine Tauzin, Jean-Christophe Aymes, Frédéric Lange, Charlotte Recapet, Jacques Rives | <p>1. During the reproductive season, animals have to manage both their energetic budget and gamete stock. In particular, for semelparous capital breeders with determinate fecundity and no parental care other than gametic investment, the depletion... | Behaviour & Ethology, Freshwater ecology, Life history | Francois-Xavier Dechaume-Moncharmont | 2020-06-04 15:18:56 | View | ||
15 Jul 2023
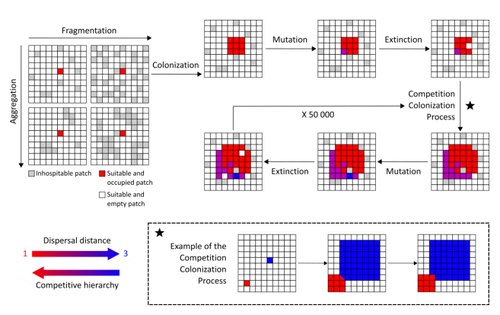
Evolution of dispersal and the maintenance of fragmented metapopulationsBasile Finand, Thibaud Monnin, Nicolas Loeuille https://doi.org/10.1101/2022.06.08.495260The spatial dynamics of habitat fragmentation drives the evolution of dispersal and metapopulation persistenceRecommended by Frédéric Guichard based on reviews by Eva Kisdi, David Murray-Stoker, Shripad Tuljapurkar and 1 anonymous reviewerThe persistence of populations facing the destruction of their habitat is a multifaceted question that has mobilized theoreticians and empiricists alike for decades. As an ecological question, persistence has been studied as the spatial rescue of populations via dispersal into remaining suitable habitats. The spatial aggregation of habitat destruction has been a key component of these studies, and it has been applied to the problem of coexistence by integrating competition-colonization tradeoffs. There is a rich ecological literature on this topic, both from theoretical and field studies (Fahrig 2003). The relationship between life-history strategies of species and their resilience to spatially structured habitat fragmentation is also an important component of conservation strategies through the management of land use, networks of protected areas, and the creation of corridors. In the context of environmental change, the ability of species to adapt to changes in landscape configuration and availability can be treated as an eco-evolutionary process by considering the possibility of evolutionary rescue (Heino and Hanski 2001; Bell 2017). However, eco-evolutionary dynamics considering spatially structured changes in landscapes and life-history tradeoffs remains an outstanding question. Finand et al. (2023) formulate the problem of persistence in fragmented landscapes over evolutionary time scales by studying models for the evolution of dispersal in relation to habitat fragmentation and spatial aggregation. Their simulations were conducted on a spatial grid where individuals can colonize suitable patch as a function of their competitive rank that decreases as a function of their (ii) dispersal distance trait. Simulations were run under fixed habitat fragmentation (proportion of unsuitable habitat) and aggregation, and with an explicit rate of habitat destruction to study evolutionary rescue. Their results reveal a balance between the selection for high dispersal under increasing habitat fragmentation and selection for lower dispersal in response to habitat aggregation. This balance leads to the coexistence of polymorphic dispersal strategies in highly aggregated landscapes with low fragmentation where high dispersers inhabit aggregated habitats while low dispersers are found in isolated habitats. The authors then integrate the spatial rescue mechanism to the problem of evolutionary rescue in response to temporally increasing fragmentation. There they show how rapid evolution allows for evolutionary rescue through the evolution of high dispersal. They also show the limits to this evolutionary rescue to cases where both aggregation and fragmentation are not too high. Interestingly, habitat aggregation prevents evolutionary rescue by directly affecting the evolutionary potential of dispersal. The study is based on simple scenarios that ignore the complexity of relationships between dispersal, landscape properties, and species interactions. This simplicity is the strength of the study, revealing basic mechanisms that can now be tested against other life-history tradeoffs and species interactions. Finand et al. (2023) provide a novel foundation for the study of eco-evolutionary dynamics in metacommunities exposed to spatially structured habitat destruction. They point to important assumptions that must be made along the way, including the relationships between dispersal distance and fecundity (they assume a positive relationship), and the nature of life-history tradeoffs between dispersal rate and local competitive abilities.
Bell, G. 2017. Evolutionary Rescue. Annual Review of Ecology, Evolution, and Systematics 48:605–627. https://doi.org/10.1146/annurev-ecolsys-110316-023011 | Evolution of dispersal and the maintenance of fragmented metapopulations | Basile Finand, Thibaud Monnin, Nicolas Loeuille | <p>Because it affects dispersal risk and modifies competition levels, habitat fragmentation directly constrains dispersal evolution. When dispersal is traded-off against competitive ability, increased fragmentation is often expected to select high... |  | Colonization, Competition, Dispersal & Migration, Eco-evolutionary dynamics, Spatial ecology, Metacommunities & Metapopulations, Theoretical ecology | Frédéric Guichard | 2022-06-10 13:51:15 | View |
MANAGING BOARD
Julia Astegiano
Tim Coulson
Anna Eklof
Dominique Gravel
François Massol
Ben Phillips
Cyrille Violle