Assessing metacommunity processes through signatures in spatiotemporal turnover of community composition
Franck Jabot, Fabien Laroche, Francois Massol, Florent Arthaud, Julie Crabot, Maxime Dubart, Simon Blanchet, Francois Munoz, Patrice David, Thibault Datry
https://doi.org/10.1101/480335
On the importance of temporal meta-community dynamics for our understanding of assembly processes
Recommended by Werner Ulrich based on reviews by Joaquín Hortal and 2 anonymous reviewers based on reviews by Joaquín Hortal and 2 anonymous reviewers
The processes that trigger community assembly are still in the centre of ecological interest. While prior work mostly focused on spatial patterns of co-occurrence within a meta-community framework [reviewed in 1, 2] recent studies also include temporal patterns of community composition [e.g. 3, 4, 5, 6]. In this preprint [7], Franck Jabot and co-workers extend they prior approaches to quasi neutral community assembly [8, 9, 10] and develop an analytical framework of spatial and temporal diversity turnover. A simple and heuristic path model for beta diversity and an extended ecological drift model serve as starting points. The model can be seen as a counterpart to Ulrich et al. [5]. These authors implemented competitive hierarchies into their neutral meta-community model while the present paper focuses on environmental filtering. Most important, the model and parameterization of four empirical data sets on aquatic plant and animal meta-communities used by Jabot et al. returned a consistent high influence of environmental stochasticity on species turnover. Of course, this major result does not come to a surprise. As typical for this kind of models it depends also to a good deal on the initial model settings. It nevertheless makes a strong conceptual point for the importance of environmental variability over dispersal and richness effects. One interesting side effect regards the impact of richness differences (ΔS). Jabot et al. interpret this as a ‘nuisance variable’ as they do not have a stringent explanation. Of course, it might be a pure statistical bias introduced by the Soerensen metric of turnover that is normalized by richness. However, I suspect that there is more behind the ΔS effect. Richness differences are generally associated with respective differences in total abundances and introduce source – sink dynamics that inevitably shape subsequent colonization – extinction processes. It would be interesting to see whether ΔS alone is able to trigger observed patterns of community assembly and community composition. Such an analysis would require partitioning of species turnover into richness and nestedness effects [11]. I encourage Jabot et al. to undertake such an effort.
The present paper is also another call to include temporal population variability into metapopulation models for a better understanding of the dynamics and triggering of community assembly. In a next step, competitive interactions should be included into the model to infer the relative importance of both factors.
References
[1] Götzenberger, L. et al. (2012). Ecological assembly rules in plant communities—approaches, patterns and prospects. Biological reviews, 87(1), 111-127. doi: 10.1111/j.1469-185X.2011.00187.x
[2] Ulrich, W., & Gotelli, N. J. (2013). Pattern detection in null model analysis. Oikos, 122(1), 2-18. doi: 10.1111/j.1600-0706.2012.20325.x
[3] Grilli, J., Barabás, G., Michalska-Smith, M. J., & Allesina, S. (2017). Higher-order interactions stabilize dynamics in competitive network models. Nature, 548(7666), 210. doi: 10.1038/nature23273
[4] Nuvoloni, F. M., Feres, R. J. F., & Gilbert, B. (2016). Species turnover through time: colonization and extinction dynamics across metacommunities. The American Naturalist, 187(6), 786-796. doi: 10.1086/686150
[5] Ulrich, W., Jabot, F., & Gotelli, N. J. (2017). Competitive interactions change the pattern of species co‐occurrences under neutral dispersal. Oikos, 126(1), 91-100. doi: 10.1111/oik.03392
[6] Dobramysl, U., Mobilia, M., Pleimling, M., & Täuber, U. C. (2018). Stochastic population dynamics in spatially extended predator–prey systems. Journal of Physics A: Mathematical and Theoretical, 51(6), 063001. doi: 10.1088/1751-8121/aa95c7
[7] Jabot, F., Laroche, F., Massol, F., Arthaud, F., Crabot, J., Dubart, M., Blanchet, S., Munoz, F., David, P., and Datry, T. (2019). Assessing metacommunity processes through signatures in spatiotemporal turnover of community composition. bioRxiv, 480335, ver. 3 peer-reviewed and recommended by PCI Ecology. doi: 10.1101/480335
[8] Jabot, F., & Chave, J. (2011). Analyzing tropical forest tree species abundance distributions using a nonneutral model and through approximate Bayesian inference. The American Naturalist, 178(2), E37-E47. doi: 10.1086/660829
[9] Jabot, F., & Lohier, T. (2016). Non‐random correlation of species dynamics in tropical tree communities. Oikos, 125(12), 1733-1742. doi: 10.1111/oik.03103
[10] Datry, T., Bonada, N., & Heino, J. (2016). Towards understanding the organisation of metacommunities in highly dynamic ecological systems. Oikos, 125(2), 149-159. doi: 10.1111/oik.02922
[11] Baselga, A. (2010). Partitioning the turnover and nestedness components of beta diversity. Global ecology and biogeography, 19(1), 134-143. doi: 10.1111/j.1466-8238.2009.00490.x
| Assessing metacommunity processes through signatures in spatiotemporal turnover of community composition | Franck Jabot, Fabien Laroche, Francois Massol, Florent Arthaud, Julie Crabot, Maxime Dubart, Simon Blanchet, Francois Munoz, Patrice David, Thibault Datry | <p>Although metacommunity ecology has been a major field of research in the last decades, with both conceptual and empirical outputs, the analysis of the temporal dynamics of metacommunities has only emerged recently and still consists mostly of r... | 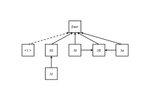 | Biodiversity, Coexistence, Community ecology, Spatial ecology, Metacommunities & Metapopulations | Werner Ulrich | | 2018-11-29 14:58:54 | View |
Crown defoliation decreases reproduction and wood growth in a marginal European beech population.
Sylvie Oddou-Muratorio, Cathleen Petit-Cailleux, Valentin Journé, Matthieu Lingrand, Jean-André Magdalou, Christophe Hurson, Joseph Garrigue, Hendrik Davi, Elodie Magnanou.
https://doi.org/10.1101/474874
Defoliation induces a trade-off between reproduction and growth in a southern population of Beech
Recommended by Georges Kunstler based on reviews by 3 anonymous reviewers
Individuals ability to withstand abiotic and biotic stresses is crucial to the maintenance of populations at climate edge of tree species distribution. We start to have a detailed understanding of tree growth response and to a lesser extent mortality response in these populations. In contrast, our understanding of the response of tree fecundity and recruitment remains limited because of the difficulty to monitor it at the individual tree level in the field. Tree recruitment limitation is, however, crucial for tree population dynamics [1-2].
In their study Oddou-Muratorio et al. [3] use a new method that they recently developed that jointly estimate male and female effective fecundity in natural populations using naturally established seedlings [4]. Their method uses a spatially explicit Bayesian analysis based on molecular markers and parentage analyses (MEMM program [4]). They apply this method to an unmanaged Beech forest at the southern edge of Beech distribution, where tree defoliation – taken as an integrative indicator of tree abiotic and biotic stress – and growth have been monitored for all adult trees.
This allows the authors to explore alternative hypothesis about tree fecundity response to stress. In one hand, biotic and abiotic stresses are thought to negatively impact tree fecundity. In the other hand, management and studies of orchard fruit tree support the idea that stress could trigger a compensatory increase of fecundity at the cost of other performances such as growth and survival.
They show that both growth and female fecundity are negatively affected by defoliation. There was no evidence that stresses trigger a compensatory increase of fecundity. Yet, they also found that, for large highly defoliated trees, there was a trade-off between growth and female fecundity. Some individuals are able to mitigate stress impact on fecundity by decreasing their growth. It is difficult to understand with available data what is driving such divergent responses between defoliated individuals. This could be related to differences in micro-environmental conditions or genetic background of individual trees. Such individual-level difference in response to stress could be crucial to understand tree populations response to ongoing climate change. This study clearly opens exciting new perspectives to apply such new methods to understand the role of fecundity on tree population dynamics. For instance, could we apply this method across the species distribution to understand how effective fecundity and its response to abiotic stress change between southern edge populations, core populations, and northern edge populations? Using time-series retrieved from such analysis can we disentangle the effect of different climatic drivers? It would also be interesting to see how such results can contribute to analyses covering the full tree life cycle to understand the contribution of fecundity response to population and evolutionary.
References
[1] Clark, J. S. et al. (1999). Interpreting recruitment limitation in forests. American Journal of Botany, 86(1), 1-16. doi: 10.2307/2656950
[2] Petit, R. J., and Hampe, A. (2006). Some evolutionary consequences of being a tree. Annu. Rev. Ecol. Evol. Syst., 37, 187-214. doi: 10.1146/annurev.ecolsys.37.091305.110215
[3] Oddou-Muratorio, S., Petit, C., Journe, V., Lingrand, M., Magdalou, J. A., Hurson, C., Garrigue, J., Davi, H. and Magnanou, E. (2019). Crown defoliation decreases reproduction and wood growth in a marginal European beech population. bioRxiv, 474874, ver. 4 peer-reviewed and recommended by PCI Ecology. doi: 10.1101/474874
[4] Oddou‐Muratorio, S. and Klein, E. K. (2008). Comparing direct vs. indirect estimates of gene flow within a population of a scattered tree species. Molecular Ecology, 17(11), 2743-2754. doi: 10.1111/j.1365-294X.2008.03783.x
| Crown defoliation decreases reproduction and wood growth in a marginal European beech population. | Sylvie Oddou-Muratorio, Cathleen Petit-Cailleux, Valentin Journé, Matthieu Lingrand, Jean-André Magdalou, Christophe Hurson, Joseph Garrigue, Hendrik Davi, Elodie Magnanou. | <p>1. Although droughts and heatwaves have been associated to increased crown defoliation, decreased growth and a higher risk of mortality in many forest tree species, their impact on tree reproduction and forest regeneration still remains underst... |  | Climate change, Eco-evolutionary dynamics, Molecular ecology, Physiology, Population ecology | Georges Kunstler | | 2018-11-20 13:29:42 | View |
Sexual segregation in a highly pagophilic and sexually dimorphic marine predator
Christophe Barbraud, Karine Delord, Akiko Kato, Paco Bustamante, Yves Cherel
https://doi.org/10.1101/472431
Sexual segregation in a sexually dimorphic seabird: a matter of spatial scale
Recommended by Denis Réale based on reviews by Dries Bonte and 1 anonymous reviewer
Sexual segregation appears in many taxa and can have important ecological, evolutionary and conservation implications. Sexual segregation can take two forms: either the two sexes specialise in different habitats but share the same area (habitat segregation), or they occupy the same habitat but form separate, unisex groups (social segregation) [1,2]. Segregation would have evolved as a way to avoid, or at least, reduce intersexual competition.
Testing whether social or habitat segregation is at play necessitates the use of combined approaches to determine the spatial scale at which segregation occurs. This enterprise is even more challenging when studying marine species, which travel over long distances to reach their foraging areas. This is what Barbraud et al. [3] have endeavoured on the snow petrel (Pagodroma nivea), a sexually dimorphic, polar seabird. Studying sexual segregation at sea requires tools for indirect measures of habitat use and foraging tactics. During the incubation period, in a colony based at Pointe Geologie, Adelie land, East Antarctica, the team has equipped birds with GPS loggers to analyse habitat use and foraging behaviour. It has also compared short-, mid-, and long-term stable isotopic profiles, from plasma, blood cells, and feather samples, respectively.
Barbraud et al. [3] could not detect any evidence for sexual segregation in space use. Furthermore, the two sexes showed similar δ13C profiles, illustrating similar foraging latitudes, and indicating no sexual segregation at large spatial scales. Snow petrels all forage exclusively in the sea ice environment formed over the deep Antarctic continental shelf. The authors, however, found other forms of segregation: males consistently foraged at higher sea ice concentrations than females. Males also fed on higher trophic levels than females. Therefore, male and female snow petrels segregate at a smaller spatial scale, and use different foraging tactics and diet specialisations. Females also took shorter foraging trips than males, with higher mass gain that strongly benefit from higher sea ice concentration. Mass gain in males increased with the length of their foraging trip at sea ice areas.
The authors conclude that high sea ice concentration offers the most favourable foraging habitat for snow petrels, and thus that intersexual competition may drive females away from high sea ice areas. This study shows that combining information from different tools provides an elegant way of isolating the potential factors driving sexual segregation and the spatial scales at which it occurs.
References
[1] Conradt, L. (2005). Definitions, hypotheses, models and measures in the study of animal segregation. In Sexual segregation in vertebrates: ecology of the two sexes (Ruckstuhl K.E. and Neuhaus, P. eds). Cambridge University Press, Cambridge, United Kingdom. Pp:11–34.
[2] Ruckstuhl, K. E. (2007). Sexual segregation in vertebrates: proximate and ultimate causes. Integrative and Comparative Biology, 47(2), 245-257. doi: 10.1093/icb/icm030
[3] Barbraud, C., Delord, K., Kato, A., Bustamante, P., & Cherel, Y. (2018). Sexual segregation in a highly pagophilic and sexually dimorphic marine predator. bioRxiv, 472431, ver. 3 peer-reviewed and recommended bt PCI Ecology. doi: 10.1101/472431
| Sexual segregation in a highly pagophilic and sexually dimorphic marine predator | Christophe Barbraud, Karine Delord, Akiko Kato, Paco Bustamante, Yves Cherel | <p>Sexual segregation is common in many species and has been attributed to intra-specific competition, sex-specific differences in foraging efficiency or in activity budgets and habitat choice. However, very few studies have simultaneously quantif... |  | Foraging, Marine ecology | Denis Réale | Dries Bonte, Anonymous | 2018-11-19 13:40:59 | View |
Evaluating functional dispersal and its eco-epidemiological implications in a nest ectoparasite
Amalia Rataud, Marlène Dupraz, Céline Toty, Thomas Blanchon, Marion Vittecoq, Rémi Choquet, Karen D. McCoy
https://doi.org/10.5281/zenodo.2592114
Limited dispersal in a vector on territorial hosts
Recommended by Adele Mennerat based on reviews by Shelly Lachish and 1 anonymous reviewer
Parasitism requires parasites and hosts to meet and is therefore conditioned by their respective dispersal abilities. While dispersal has been studied in a number of wild vertebrates (including in relation to infection risk), we still have poor knowledge of the movements of their parasites. Yet we know that many parasites, and in particular vectors transmitting pathogens from host to host, possess the ability to move actively during at least part of their lives.
So... how far does a vector go – and is this reflected in the population structure of the pathogens they transmit? This is the question addressed by Rataud et al. [1], who provide the first attempt at using capture-mark-recapture to estimate not only functional dispersal, but also detection probability and survival in a wild parasite that is also a vector for other pathogens.
The authors find that (i) functional dispersal of soft ticks within a gull colony is very limited. Moreover, they observe unexpected patterns: (ii) experimental displacement of ticks does not induce homing behaviour, and (iii) despite lower survival, tick dispersal was lower in nests not containing hosts than in successful nests.
These results contrast with expectations based on the distribution of infectious agents. Low tick dispersal within the colony, combined with host territoriality during breeding and high site fidelity between years should result in a spatially structured distribution of infectious agents carried by ticks. This is not the case here. One possible explanation could be that soft ticks live for much longer than a breeding season, and that they disperse at other times of year to a larger extent than usually assumed.
This study represents one chapter of a story that will likely keep unfolding. It raises fascinating questions, and illustrates the importance of basic knowledge of parasite ecology and behaviour to better understand pathogen dynamics in the wild.
References
[1] Rataud A., Dupraz M., Toty C., Blanchon T., Vittecoq M., Choquet R. & McCoy K.D. (2019). Evaluating functional dispersal and its eco-epidemiological implications in a nest ectoparasite. Zenodo, 2592114. Ver. 3 peer-reviewed and recommended by PCI Ecology. doi: 10.5281/zenodo.2592114
| Evaluating functional dispersal and its eco-epidemiological implications in a nest ectoparasite | Amalia Rataud, Marlène Dupraz, Céline Toty, Thomas Blanchon, Marion Vittecoq, Rémi Choquet, Karen D. McCoy | <p>Functional dispersal (between-site movement, with or without subsequent reproduction) is a key trait acting on the ecological and evolutionary trajectories of a species, with potential cascading effects on other members of the local community. ... |  | Dispersal & Migration, Epidemiology, Parasitology, Population ecology | Adele Mennerat | | 2018-11-05 11:44:58 | View |
Interplay between the paradox of enrichment and nutrient cycling in food webs
Pierre Quévreux, Sébastien Barot and Élisa Thébault
https://doi.org/10.1101/276592
New insights into the role of nutrient cycling in food web dynamics
Recommended by Samraat Pawar based on reviews by Jean-François Arnoldi, Wojciech Uszko and 1 anonymous reviewer based on reviews by Jean-François Arnoldi, Wojciech Uszko and 1 anonymous reviewer
Understanding the factors that govern the relationship between structure, stability and functioning of food webs has been a central problem in ecology for many decades. Historically, apart from microbial and soil food webs, the role of nutrient cycling has largely been ignored in theoretical and empirical food web studies. A prime example of this is the widespread use of Lotka-Volterra type models in theoretical studies; these models per se are not designed to capture the effect of nutrients being released back into the system by interacting populations. Thus overall, we still lack a general understanding of how nutrient cycling affects food web dynamics.
A new study by Quévreux, Barot and Thébault [1] tackles this problem by building a new food web model. This model features some important biological details: trophic interactions and vital rates constrained by species' body masses (using Ecological Metabolic Theory), adaptive foraging, and stoichiometric rules to ensure meaningful conversion between carbon and nutrient flows. The authors analyze the model through detailed simulations combined with thorough sensitivity analyses of model assumptions and parametrizations (including of allometric scaling relationships). I am happy to recommend this preprint because of the novelty of the work and it's technical quality.
The study yields interesting and novel findings. Overall, nutrient cycling does have a strong effect on community dynamics. Nutrient recycling is driven mostly by consumers at low mineral nutrient inputs, and by primary producers at high inputs. The extra nutrients made available through recycling increases species' persistence at low nutrient input levels, but decreases persistence at higher input levels by increasing population oscillations (a new, nuanced perspective on the classical "paradox of enrichment"). Also, for the same level of nutrient input, food webs with nutrient recycling show more fluctuations in primary producer biomass (and less at higher trophic levels) than those without recycling, with this effect weakening in more complex food webs.
Overall, these results provide new insights, suggesting that nutrient cycling may enhance the positive effects of species richness on ecosystem stability, and point at interesting new directions for future theoretical and empirical studies.
References
[1] Quévreux, P., Barot, S. and E. Thébault (2020) Interplay between the paradox of enrichment and nutrient cycling in food webs. bioRxiv, 276592, ver. 7 peer-reviewed and recommended by PCI Ecology. doi: 10.1101/276592
| Interplay between the paradox of enrichment and nutrient cycling in food webs | Pierre Quévreux, Sébastien Barot and Élisa Thébault | <p>Nutrient cycling is fundamental to ecosystem functioning. Despite recent major advances in the understanding of complex food web dynamics, food web models have so far generally ignored nutrient cycling. However, nutrient cycling is expected to ... |  | Biodiversity, Community ecology, Ecosystem functioning, Food webs, Interaction networks, Theoretical ecology | Samraat Pawar | | 2018-11-03 21:47:37 | View |
Understanding geographic range expansions in human-dominated landscapes: does behavioral flexibility modulate flexibility in foraging and social behavior?
Recommended by Julia Astegiano and Esther Sebastián González and Esther Sebastián González based on reviews by Pizza Ka Yee Chow and Esther Sebastián González based on reviews by Pizza Ka Yee Chow and Esther Sebastián González
Which biological traits modulate species distribution has historically been and still is one of the core questions of the macroecology and biogeography agenda [1, 2]. As most of the Earth surface has been modified by human activities [3] understanding the strategies that allow species to inhabit human-dominated landscapes will be key to explain species geographic distribution in the Anthropocene. In this vein, Logan et al. [4] are working on a long-term and integrative project aimed to investigate how great-tailed grackles rapidly expanded their geographic range into North America [4]. Particularly, they want to determine which is the role of behavioral flexibility, i.e. an individual’s ability to modify its behavior when circumstances change based on learning from previous experience [5], in rapid geographic range expansions. The authors are already working in a set of complementary questions described in pre-registrations that have already been recommended at PCI Ecology: (1) Do individuals with greater behavioral flexibility rely more on causal cognition [6]? (2) Which are the mechanisms that lead to behavioral flexibility [7]? (3) Does the manipulation of behavioral flexibility affect exploration, but not boldness, persistence, or motor diversity [8]? (4) Can context changes improve behavioral flexibility [9]?
In this new pre-registration, they aim to determine whether the more behaviorally flexible individuals have more flexible foraging behaviors (i.e. use a wider variety of foraging techniques in the wild and eat a larger number of different foods), habitat use (i.e. higher microhabitat richness) and social relationships (i.e., are more likely to have a greater number of bonds or stronger bonds with other individuals; [4]). The project is ambitious, combining both the experimental characterization of individuals’ behavioral flexibility and the field characterization of the foraging and social behavior of those individuals and of wild ones.
The current great-tailed grackles project will be highly relevant to understand rapid geographic range expansions in a changing world. In this vein, this pre-registration will particularly help to go one step further in our understanding of behavioral flexibility as a determinant of species geographic distribution. Logan et al. [4] pre-registration is very well designed, main and alternative hypotheses have been thought and written and methods are presented in a very detailed way, which includes the R codes that authors will use in their analyses. Authors have answered in a very detailed way each comment that reviewers have pointed out and modified the pre-registration accordingly, which we consider highly improved the quality of this work. That is why we strongly recommend this pre-registration and look forward to see the results.
References
[1] Gaston K. J. (2003) The structure and dynamics of geographic ranges. Oxford series in Ecology and Evolution. Oxford University Press, New York.
[2] Castro-Insua, A., Gómez‐Rodríguez, C., Svenning, J.C., and Baselga, A. (2018) A new macroecological pattern: The latitudinal gradient in species range shape. Global ecology and biogeography, 27(3), 357-367. doi: 10.1111/geb.12702
[3] Newbold, T., Hudson, L. N., Hill, S. L. L., Contu, S., Lysenko, I., Senior, R. A., et al. (2015). Global effects of land use on local terrestrial biodiversity. Nature, 520(7545), 45–50. doi: 10.1038/nature14324
[4] Logan CJ, McCune K, Bergeron L, Folsom M, Lukas D. (2019). Is behavioral flexibility related to foraging and social behavior in a rapidly expanding species? In principle recommendation by Peer Community In Ecology. http://corinalogan.com/Preregistrations/g_flexforaging.html
[5] Mikhalevich, I., Powell, R., and Logan, C. (2017). Is Behavioural Flexibility Evidence of Cognitive Complexity? How Evolution Can Inform Comparative Cognition. Interface Focus 7: 20160121. doi: 10.1098/rsfs.2016.0121.
[6] Fronhofer, E. (2019) From cognition to range dynamics: advancing our understanding of macroecological patterns. Peer Community in Ecology, 100014. doi: 10.24072/pci.ecology.100014
[7] Vogel, E. (2019) Adapting to a changing environment: advancing our understanding of the mechanisms that lead to behavioral flexibility. Peer Community in Ecology, 100016. doi: 10.24072/pci.ecology.100016
[8] Van Cleve, J. (2019) Probing behaviors correlated with behavioral flexibility. Peer Community in Ecology, 100020. doi: 10.24072/pci.ecology.100020
[9] Coulon, A. (2019) Can context changes improve behavioral flexibility? Towards a better understanding of species adaptability to environmental changes. Peer Community in Ecology, 100019. doi: 10.24072/pci.ecology.100019
| Is behavioral flexibility related to foraging and social behavior in a rapidly expanding species? | Corina Logan, Luisa Bergeron, Carolyn Rowney, Kelsey McCune, Dieter Lukas | This is one of the first studies planned for our long-term research on the role of behavioral flexibility in rapid geographic range expansions. Project background: Behavioral flexibility, the ability to change behavior when circumstances change ba... |  | Behaviour & Ethology, Preregistrations, Zoology | Julia Astegiano | | 2018-10-23 00:47:03 | View |
Direct and transgenerational effects of an experimental heat wave on early life stages in a freshwater snail
Katja Leicht, Otto Seppälä
https://doi.org/10.1101/449777
Escargots cooked just right: telling apart the direct and indirect effects of heat waves in freashwater snails
Recommended by vincent calcagno based on reviews by Amanda Lynn Caskenette, Kévin Tougeron and arnaud sentis
Amongst the many challenges and forms of environmental change that organisms face in our era of global change, climate change is perhaps one of the most straightforward and amenable to investigation. First, measurements of day-to-day temperatures are relatively feasible and accessible, and predictions regarding the expected trends in Earth surface temperature are probably some of the most reliable we have. It appears quite clear, in particular, that beyond the overall increase in average temperature, the heat waves locally experienced by organisms in their natural habitats are bound to become more frequent, more intense, and more long-lasting [1]. Second, it is well appreciated that temperature is a major environmental factor with strong impacts on different facets of organismal development and life-history [2-4]. These impacts have reasonably clear mechanistic underpinnings, with definite connections to biochemistry, physiology, and considerations on energetics. Third, since variation in temperature is a challenge already experienced by natural populations across their current and historical ranges, it is not a completely alien form of environmental change. Therefore, we already learnt quite a lot about it in several species, and so did the species, as they may be expected to have evolved dedicated adaptive mechanisms to respond to elevated temperatures. Last, but not least, temperature is quite amenable to being manipulated as an experimental factor.
For all these reasons, experimental studies of the consequences of increased temperature hit some of a sweetspot and are a source of very nice research, in many different organisms. The work by Leicht and Seppala [5] complements a sequence of earlier studies by this group, using the freshwater snail Lymnaea stagnalis as their model system [6-7].
In the present study, the authors investigate how a heat wave (a period of abnormally elevated temperature, here 25°C versus a normal 15°C) may have indirect effects on the next generation, through maternal effects. They question whether such indirect effects exist, and if they exist, how they compare, in terms of effect size, with the (more straightforward) direct effects observed in individuals that directly experience a heat wave. Transgenerational effects are well-known to occur following periods of physiological stress, and might thus have non negligible contributions to the overall effect of warming.
In this freshwater snail, heat has very strong direct effects: mortality increases at high temperature, but survivors grow much bigger, with a greater propensity to lay eggs and a (spectacular) three-fold increase in the number of eggs laid [6]. Considering that, it is easy to consider that transgenerational effects should be small game. And indeed, the present study also observes the big and obvious direct effects of elevated temperature: higher mortality, but greater propensity to oviposit. However, it was also found that the eggs were smaller if from mothers exposed to high temperature, with a correspondingly smaller size of hatchlings. This suggests that a heat wave causes the snails to lay more eggs, but smaller ones, reminiscent of a size-number trade-off. Unfortunately, clutch size could not be measured in this experiment, so this cannot be investigated any further. For this trait, the indirect effect may indeed be regarded as small game : eggs and hatchlings were about 15 % smaller, an effect size pretty small compared to the mammoth direct positive effect of temperature on shell length (see Figure 4 ; and also [6]). The same is true for developmental time (Figure 3).
However, for some traits the story was different. In particular, it was found that the (smaller) eggs produced from heated mothers were more likely to hatch by almost 10% (Figure 2). Here the indirect effect not only goes against the direct effect (hatching rate is lower at high temperature), but it also has similar effect size. As a consequence, taking into account both the indirect and direct effects, hatching success is essentially the same at 15°C and 25°C (Figure 2). Survival also had comparable effect sizes for direct and indirect effects. Indeed, survival was reduced by about 20% regardless of whom endured the heat stress (the focal individual or her mother; Figure 4). Interestingly, the direct and indirect effects were not quite cumulative: if a mother experienced a heat wave, heating up the offspring did not do much more damage, as though the offspring were ‘adapted’ to the warmer conditions (but keep in mind that, surprisingly, the authors’ stats did not find a significant interaction; Table 2).
At the end of the day, even though at first heat seems a relatively simple and understandable component of environmental change, this study shows how varied its effects can be effects on different components of individual fitness. The overall impact most likely is a mix of direct and indirect effects, of shifts along allocation trade-offs, and of maladaptive and adaptive responses, whose overall ecological significance is not so easy to grasp. That said, this study shows that direct and indirect (maternal) effects can sometimes go against one another and have similar intensities. Indirect effects should therefore not be overlooked in this kind of studies. It also gives a hint of what an interesting challenge it is to understand the adaptive or maladaptive nature of organism responses to elevated temperatures, and to evaluate their ultimate fitness consequences.
References
[1] Meehl, G. A., & Tebaldi, C. (2004). More intense, more frequent, and longer lasting heat waves in the 21st century. Science (New York, N.Y.), 305(5686), 994–997. doi: 10.1126/science.1098704
[2] Adamo, S. A., & Lovett, M. M. E. (2011). Some like it hot: the effects of climate change on reproduction, immune function and disease resistance in the cricket Gryllus texensis. The Journal of Experimental Biology, 214(Pt 12), 1997–2004. doi: 10.1242/jeb.056531
[3] Deutsch, C. A., Tewksbury, J. J., Tigchelaar, M., Battisti, D. S., Merrill, S. C., Huey, R. B., & Naylor, R. L. (2018). Increase in crop losses to insect pests in a warming climate. Science (New York, N.Y.), 361(6405), 916–919. doi: 10.1126/science.aat3466
[4] Sentis, A., Hemptinne, J.-L., & Brodeur, J. (2013). Effects of simulated heat waves on an experimental plant–herbivore–predator food chain. Global Change Biology, 19(3), 833–842. doi: 10.1111/gcb.12094
[5] Leicht, K., & Seppälä, O. (2019). Direct and transgenerational effects of an experimental heat wave on early life stages in a freshwater snail. BioRxiv, 449777, ver. 4 peer-reviewed and recommended by PCI Ecology. doi: 10.1101/449777
[6] Leicht, K., Seppälä, K., & Seppälä, O. (2017). Potential for adaptation to climate change: family-level variation in fitness-related traits and their responses to heat waves in a snail population. BMC Evolutionary Biology, 17(1), 140. doi: 10.1186/s12862-017-0988-x
[7] Leicht, K., Jokela, J., & Seppälä, O. (2013). An experimental heat wave changes immune defense and life history traits in a freshwater snail. Ecology and Evolution, 3(15), 4861–4871. doi: 10.1002/ece3.874
| Direct and transgenerational effects of an experimental heat wave on early life stages in a freshwater snail | Katja Leicht, Otto Seppälä | <p>Global climate change imposes a serious threat to natural populations of many species. Estimates of the effects of climate change‐mediated environmental stresses are, however, often based only on their direct effects on organisms, and neglect t... |  | Climate change | vincent calcagno | | 2018-10-22 22:19:22 | View |
Differential immune gene expression associated with contemporary range expansion of two invasive rodents in Senegal
Nathalie Charbonnel, Maxime Galan, Caroline Tatard, Anne Loiseau, Christophe Diagne, Ambroise Dalecky, Hugues Parrinello, Stephanie Rialle, Dany Severac and Carine Brouat
https://doi.org/10.1101/442160
Are all the roads leading to Rome?
Recommended by Simon Blanchet based on reviews by Nadia Aubin-Horth and 1 anonymous reviewer
Identifying the factors which favour the establishment and spread of non-native species in novel environments is one of the keys to predict - and hence prevent or control - biological invasions. This includes biological factors (i.e. factors associated with the invasive species themselves), and one of the prevailing hypotheses is that some species traits may explain their impressive success to establish and spread in novel environments [1]. In animals, most research studies have focused on traits associated with fecundity, age at maturity, level of affiliation to humans or dispersal ability for instance. The “composite picture” of the perfect (i.e. successful) invader that has gradually emerged is a small-bodied animal strongly affiliated to human activities with high fecundity, high dispersal ability and a super high level of plasticity. Of course, the story is not that simple, and actually a perfect invader sometimes – if not often- takes another form… Carrying on to identify what makes a species a successful invader or not is hence still an important research axis with major implications.
In this manuscript, Charbonnel and collaborators [2] provide an interesting opportunity to gain novel insights into our understanding of (the) traits underlying invasion success. They nicely combine the power of Next-Generation Sequencing (NGS) with a clever comparative approach of two closely-related invasive rodents (the house mouse Mus musculus and the black rat Rattus rattus) in a common environment. They use this experimental design to test the appealing hypothesis that pathogens may be actors of the story, and may indirectly explain why some non-native species are so successful in invading novel habitats.
It is generally assumed that the community of pathogens encountered by non-native species in novel environments is different from that of their native area. On the one hand (the enemy-release hypothesis), it can be hypothesized that non-native species, when they arrive into a novel environment, will be relaxed from the pressure imposed by their native pathogens because local pathogens are not adapted (and hence do not infect) to this novel host. Because immune defence against pathogens is highly costly, non-native species establishing into a novel environment could hence reallocate these costs to other functions such as fecundity or dispersal apparatus. This scenario has been termed the “evolution of increased competitive ability” (EICA) hypothesis [3]. On the other hand (the EICA-refined hypothesis [4]), one can assume that invaders will encounter new pathogens in newly established areas, and will allocate energy toward cost-effective immune pathways to permit allocating a non-negligible amount of energy toward other functions. Finally, a last hypothesis (the “immune protection” hypothesis) assumes major changes in pathogen composition between native and invaded areas, which should lead to an overall increase in immune investment by the native species to successfully invade novel environments [4]. This last hypothesis suggests that only non-native species being able to take up the associated costs of immunity will be successful invaders.
The role of immunity in invasion success has yet been poorly investigated, mainly because of the difficulty to simultaneously analyse multiple immune pathways [4]. Charbonnel and collaborators [2] overpass this difficulty by screening all genes expressed (using a whole RNA sequencing approach) in an immune tissue: the spleen. They do so along the invasion routes of two sympatric invasive rodents in Africa and compare anciently and newly invaded areas (respectively). For one of the two species (the house mouse), they found a high number of immune-related genes to be up-regulated in newly invaded areas compared to anciently invaded areas. All categories of immune pathways (costly and cost-effective) were up-regulated, suggesting an overall increase in immune investment in the mouse, which corroborates the “immune protection” hypothesis. For the black rat, patterns of gene expression were somewhat different, with much less pronounced differentiation in gene expression between newly and anciently invaded areas. Among the few differentiated genes, a few were associated to immune responses and some of theses genes were even down-regulated in the newly invaded areas. This pattern may actually corroborate the EICA hypothesis, although it could alternatively suggest that stochastic processes (drift) associated to recent decrease in population size (which is expected during a colonisation event) are more important than selection imposed by pathogens in shaping patterns of immune gene expression.
Overall, this study [2] suggests (i) that immune-related traits are important in predicting invasion success and (ii) that two successful species with a similar invasion history and living in similar environments can use different life-history strategies to reach the same success. This later finding is particularly relevant and intriguing as it suggests that the traits and strategies deployed by species to colonise new habitats might actually be idiosyncratic, and that, if general trends actually emerge in regards of traits predicting the success of invaders, the devil might actually be into the details. Comparative studies are extremely important to identify the general rules and the specificities sustaining actual patterns, but these approaches are yet poorly used in biological invasions (at least empirically). The work presented by Charbonnel and colleagues [2] calls for future comparative studies performed at multiple spatial scales (native vs. non-native areas, anciently vs. recently invaded areas), multiple taxonomic resolutions and across multiple traits (to search for trade-offs), so that the success of invasive species can be properly understood and predicted.
References
[1] Jeschke, J. M., & Strayer, D. L. (2006). Determinants of vertebrate invasion success in Europe and North America. Global Change Biology, 12(9), 1608-1619. doi: 10.1111/j.1365-2486.2006.01213.x
[2] Blossey, B., & Notzold, R. (1995). Evolution of increased competitive ability in invasive nonindigenous plants: a hypothesis. Journal of Ecology, 83(5), 887-889. doi: 10.2307/2261425
[3] Charbonnel, N., Galan, M., Tatard, C., Loiseau, A., Diagne, C. A., Dalecky, A., Parrinello, H., Rialle, S., Severac, D., & Brouat, C. (2019). Differential immune gene expression associated with contemporary range expansion of two invasive rodents in Senegal. bioRxiv, 442160, ver. 5 peer-reviewed and recommended by PCI Ecology. doi: 10.1101/442160
[4] Lee, K. A., & Klasing, K. C. (2004). A role for immunology in invasion biology. Trends in Ecology & Evolution, 19(10), 523-529. doi: 10.1016/j.tree.2004.07.012
| Differential immune gene expression associated with contemporary range expansion of two invasive rodents in Senegal | Nathalie Charbonnel, Maxime Galan, Caroline Tatard, Anne Loiseau, Christophe Diagne, Ambroise Dalecky, Hugues Parrinello, Stephanie Rialle, Dany Severac and Carine Brouat | <p>Background: Biological invasions are major anthropogenic changes associated with threats to biodiversity and health. What determines the successful establishment of introduced populations still remains unsolved. Here we explore the appealing as... |  | Biological invasions, Eco-immunology & Immunity, Population ecology | Simon Blanchet | | 2018-10-14 12:21:52 | View |
Adapting to a changing environment: advancing our understanding of the mechanisms that lead to behavioral flexibility
Recommended by Erin Vogel based on reviews by Simon Gingins and 2 anonymous reviewers
Behavioral flexibility is essential for organisms to adapt to an ever-changing environment. However, the mechanisms that lead to behavioral flexibility and understanding what traits makes a species better able to adapt behavior to new environments has been understudied. Logan and colleagues have proposed to use a series of experiments, using great-tailed grackles as a study species, to test four main hypotheses. These hypotheses are centered around exploring the relationship between behavioral flexibility and inhibition in grackles. This current preregistration is a part of a larger integrative research plan examining behavioral flexibility when faced with environmental change. In this part of the project they will examine specifically if individuals that are more flexible are also better at inhibiting: in other words: they will test the assumption that inhibition is required for flexibility.
First, they will test the hypothesis that behavioral flexibility is manipulatable by using a serial reversal learning task. Second, they will test the hypothesis that manipulating behavioral flexibility (improving reversal learning speed through serial reversals using colored tubers) improves flexibility (rule switching) and problem solving in a new context (multi‑access box and serial reversals on a touch screen). Third, they will test the hypothesis that behavioral flexibility within a context is repeatable within individuals, which is important to test if performance is state dependent. Finally, they will test a fourth hypothesis that individuals should converge on an epsilon‑first learning strategy (learn the correct choice after one trial) as they progress through serial reversals. Their innovative approach using three main tasks (delay of gratification, go-no, detour) will allow them to assess different aspects of inhibitory control. They will analyze the results of all three experiments to also assess the utility of these experiments for studying the potential relationship between inhibition and behavioral flexibility.
In their preregistration, Logan and colleagues have proposed to test these hypotheses, each with a set of testable predictions that can be examined with detailed and justified methodologies. They have also provided a comprehensive plan for analyzing the data. All of the reviewers and I agree that this is a very interesting study that has the potential to answer important questions about a critical topic in behavioral ecology: the role of inhibition in the evolution of behavioral flexibility. Given the positive reviews, the comprehensive responses by the PI and her colleagues, and careful revisions, I highly recommend this preregistration.
| Are the more flexible great-tailed grackles also better at inhibition? | Corina Logan, Kelsey McCune, Zoe Johnson-Ulrich, Luisa Bergeron, Carolyn Rowney, Benjamin Seitz, Aaron Blaisdell, Claudia Wascher | This is a PREREGISTRATION. The DOI was issued by OSF and refers to the whole GitHub repository, which contains multiple files. The specific file we are submitting is g_inhibition.Rmd, which is easily accessible at GitHub at https://github.com/cori... |  | Behaviour & Ethology, Preregistrations, Zoology | Erin Vogel | | 2018-10-12 18:36:00 | View |
Using a large-scale biodiversity monitoring dataset to test the effectiveness of protected areas at conserving North-American breeding birds
Victor Cazalis, Soumaya Belghali, Ana S.L. Rodrigues
https://doi.org/10.1101/433037
Protected Areas effects on biodiversity: a test using bird data that hopefully will give ideas for much more studies to come
Recommended by Paul Caplat based on reviews by Willson Gaul and 1 anonymous reviewer
In the face of worldwide declines in biodiversity, evaluating the effectiveness of conservation practices is an absolute necessity. Protected Areas (PA) are a key tool for conservation, and the question “Are PA effective” has been on many a research agenda, as the introduction to this preprint will no doubt convince you. A challenge we face is that, until now, few studies have been explicitly designed to evaluate PA, and despite the rise of meta-analyses on the topic, our capacity to quantify their effect on biodiversity remains limited.
This study by Cazalis et al. [1] uses the rich dataset of the North-American Breeding Bird Survey and a sound paired design to investigate how PA change bird assemblages. The methodological care brought to the study in itself is worth the read, and the results are insightful. I will not spoil too much by revealing here that things are “complicated”, and that effects – or lack thereof – depend on the type of ecosystem, and the type of species considered.
If you are interested in conservation, bird communities, species life-history, or like beautiful plots: go and read it.
References
[1] Cazalis, V., Belghali, S., & Rodrigues, A. S. (2019). Using a large-scale biodiversity monitoring dataset to test the effectiveness of protected areas at conserving North-American breeding birds. bioRxiv, 433037, ver. 4 peer-reviewed and recommended by PCI Ecology. doi: 10.1101/433037
| Using a large-scale biodiversity monitoring dataset to test the effectiveness of protected areas at conserving North-American breeding birds | Victor Cazalis, Soumaya Belghali, Ana S.L. Rodrigues | <p>Protected areas currently cover about 15% of the global land area, and constitute one of the main tools in biodiversity conservation. Quantifying their effectiveness at protecting species from local decline or extinction involves comparing prot... |  | Biodiversity, Conservation biology, Human impact, Landscape ecology, Macroecology | Paul Caplat | | 2018-10-04 08:43:34 | View |



 based on reviews by Joaquín Hortal and 2 anonymous reviewers
based on reviews by Joaquín Hortal and 2 anonymous reviewers







 based on reviews by Jean-François Arnoldi, Wojciech Uszko and 1 anonymous reviewer
based on reviews by Jean-François Arnoldi, Wojciech Uszko and 1 anonymous reviewer
 and Esther Sebastián González
and Esther Sebastián González based on reviews by Pizza Ka Yee Chow and Esther Sebastián González
based on reviews by Pizza Ka Yee Chow and Esther Sebastián González
















