Latest recommendations

| Id | Title | Authors | Abstract | Picture | Thematic fields▲ | Recommender | Reviewers | Submission date | |
|---|---|---|---|---|---|---|---|---|---|
16 Sep 2019
Blood, sweat and tears: a review of non-invasive DNA samplingMarie-Caroline Lefort, Robert H Cruickshank, Kris Descovich, Nigel J Adams, Arijana Barun, Arsalan Emami-Khoyi, Johnaton Ridden, Victoria R Smith, Rowan Sprague, Benjamin Waterhouse, Stephane Boyer https://doi.org/10.1101/385120Words matter: extensive misapplication of "non-invasive" in describing DNA sampling methods, and proposed clarifying termsRecommended by Thomas Wilson Sappington based on reviews by 2 anonymous reviewersThe ability to successfully sequence trace quantities of environmental DNA (eDNA) has provided unprecedented opportunities to use genetic analyses to elucidate animal ecology, behavior, and population structure without affecting the behavior, fitness, or welfare of the animal sampled. Hair associated with an animal track in the snow, the shed exoskeleton of an insect, or a swab of animal scat are all examples of non-invasive methods to collect eDNA. Despite the seemingly uncomplicated definition of "non-invasive" as proposed by Taberlet et al. [1], Lefort et al. [2] highlight that its appropriate application to sampling methods in practice is not so straightforward. For example, collecting scat left behind on the forest floor by a mammal could be invasive if feces is used by that species to mark territorial boundaries. Other collection strategies such as baited DNA traps to collect hair, capturing and handling an individual to swab or stimulate emission of a body fluid, or removal of a presumed non essential body part like a feather, fish scale, or even a leg from an insect are often described as "non-invasive" sampling methods. However, such methods cannot be considered truly non-invasive. At a minimum, attracting or capturing and handling an animal to obtain a DNA sample interrupts its normal behavioral routine, but additionally can cause both acute and long-lasting physiological and behavioral stress responses and other effects. Even invertebrates exhibit long-term hypersensitization after an injury, which manifests as heightened vigilance and enhanced escape responses [3-5]. References [1] Taberlet P., Waits L. P. and Luikart G. 1999. Noninvasive genetic sampling: look before you leap. Trends Ecol. Evol. 14: 323-327. doi: 10.1016/S0169-5347(99)01637-7 | Blood, sweat and tears: a review of non-invasive DNA sampling | Marie-Caroline Lefort, Robert H Cruickshank, Kris Descovich, Nigel J Adams, Arijana Barun, Arsalan Emami-Khoyi, Johnaton Ridden, Victoria R Smith, Rowan Sprague, Benjamin Waterhouse, Stephane Boyer | <p>The use of DNA data is ubiquitous across animal sciences. DNA may be obtained from an organism for a myriad of reasons including identification and distinction between cryptic species, sex identification, comparisons of different morphocryptic ... |  | Behaviour & Ethology, Conservation biology, Molecular ecology, Zoology | Thomas Wilson Sappington | 2018-11-30 13:33:31 | View | |
02 Oct 2018
How optimal foragers should respond to habitat changes? On the consequences of habitat conversion.Vincent Calcagno, Frederic Hamelin, Ludovic Mailleret, Frederic Grognard 10.1101/273557Optimal foraging in a changing world: old questions, new perspectivesRecommended by Francois-Xavier Dechaume-Moncharmont based on reviews by Frederick Adler, Andrew Higginson and 1 anonymous reviewer based on reviews by Frederick Adler, Andrew Higginson and 1 anonymous reviewer
Marginal value theorem (MVT) is an archetypal model discussed in every behavioural ecology textbook. Its popularity is largely explained but the fact that it is possible to solve it graphically (at least in its simplest form) with the minimal amount of equations, which is a sensible strategy for an introductory course in behavioural ecology [1]. Apart from this heuristic value, one may be tempted to disregard it as a naive toy model. After a burst of interest in the 70's and the 80's, the once vivid literature about optimal foraging theory (OFT) has lost its momentum [2]. Yet, OFT and MVT have remained an active field of research in the parasitoidologists community, mostly because the sampling strategy of a parasitoid in patches of hosts and its resulting fitness gain are straightforward to evaluate, which eases both experimental and theoretical investigations [3]. References [1] Fawcett, T. W. & Higginson, A. D. 2012 Heavy use of equations impedes communication among biologists. Proc. Natl. Acad. Sci. 109, 11735–11739. doi: 10.1073/pnas.1205259109 | How optimal foragers should respond to habitat changes? On the consequences of habitat conversion. | Vincent Calcagno, Frederic Hamelin, Ludovic Mailleret, Frederic Grognard | The Marginal Value Theorem (MVT) provides a framework to predict how habitat modifications related to the distribution of resources over patches should impact the realized fitness of individuals and their optimal rate of movement (or patch residen... |  | Behaviour & Ethology, Dispersal & Migration, Foraging, Landscape ecology, Spatial ecology, Metacommunities & Metapopulations, Theoretical ecology | Francois-Xavier Dechaume-Moncharmont | 2018-03-05 10:42:11 | View | |
13 Mar 2021

Investigating sex differences in genetic relatedness in great-tailed grackles in Tempe, Arizona to infer potential sex biases in dispersalSevchik, A., Logan, C. J., McCune, K. B., Blackwell, A., Rowney, C. and Lukas, D https://doi.org/10.32942/osf.io/t6behDispersal: from “neutral” to a state- and context-dependent viewRecommended by Emanuel A. Fronhofer based on reviews by 2 anonymous reviewersTraditionally, dispersal has often been seen as “random” or “neutral” as Lowe & McPeek (2014) have put it. This simplistic view is likely due to dispersal being intrinsically difficult to measure empirically as well as “random” dispersal being a convenient simplifying assumption in theoretical work. Clobert et al. (2009), and many others, have highlighted how misleading this assumption is. Rather, dispersal seems to be usually a complex reaction norm, depending both on internal as well as external factors. One such internal factor is the sex of the dispersing individual. A recent review of the theoretical literature (Li & Kokko 2019) shows that while ideas explaining sex-biased dispersal go back over 40 years this state-dependency of dispersal is far from comprehensively understood. Sevchik et al. (2021) tackle this challenge empirically in a bird species, the great-tailed grackle. In contrast to most bird species, where females disperse more than males, the authors report genetic evidence indicating male-biased dispersal. The authors argue that this difference can be explained by the great-tailed grackle’s social and mating-system. Dispersal is a central life-history trait (Bonte & Dahirel 2017) with major consequences for ecological and evolutionary processes and patterns. Therefore, studies like Sevchik et al. (2021) are valuable contributions for advancing our understanding of spatial ecology and evolution. Importantly, Sevchik et al. also lead to way to a more open and reproducible science of ecology and evolution. The authors are among the pioneers of preregistering research in their field and their way of doing research should serve as a model for others. References Bonte, D. & Dahirel, M. (2017) Dispersal: a central and independent trait in life history. Oikos 126: 472-479. doi: https://doi.org/10.1111/oik.03801 Clobert, J., Le Galliard, J. F., Cote, J., Meylan, S. & Massot, M. (2009) Informed dispersal, heterogeneity in animal dispersal syndromes and the dynamics of spatially structured populations. Ecol. Lett.: 12, 197-209. doi: https://doi.org/10.1111/j.1461-0248.2008.01267.x Li, X.-Y. & Kokko, H. (2019) Sex-biased dispersal: a review of the theory. Biol. Rev. 94: 721-736. doi: https://doi.org/10.1111/brv.12475 Lowe, W. H. & McPeek, M. A. (2014) Is dispersal neutral? Trends Ecol. Evol. 29: 444-450. doi: https://doi.org/10.1016/j.tree.2014.05.009 Sevchik, A., Logan, C. J., McCune, K. B., Blackwell, A., Rowney, C. & Lukas, D. (2021) Investigating sex differences in genetic relatedness in great-tailed grackles in Tempe, Arizona to infer potential sex biases in dispersal. EcoEvoRxiv, osf.io/t6beh, ver. 5 peer-reviewed and recommended by Peer community in Ecology. doi: https://doi.org/10.32942/osf.io/t6beh | Investigating sex differences in genetic relatedness in great-tailed grackles in Tempe, Arizona to infer potential sex biases in dispersal | Sevchik, A., Logan, C. J., McCune, K. B., Blackwell, A., Rowney, C. and Lukas, D | <p>In most bird species, females disperse prior to their first breeding attempt, while males remain closer to the place they hatched for their entire lives. Explanations for such female bias in natal dispersal have focused on the resource-defense ... |  | Behaviour & Ethology, Dispersal & Migration, Zoology | Emanuel A. Fronhofer | 2020-08-24 17:53:06 | View | |
12 Oct 2019
Investigating the use of learning mechanisms in a species that is rapidly expanding its geographic rangeKelsey McCune, Richard McElreath, Corina Logan http://corinalogan.com/Preregistrations/g_sociallearning.htmlHow would variation in environmental predictability affect the use of different learning mechanisms in a social bird?Recommended by Aliza le Roux based on reviews by Matthew Petelle and 1 anonymous reviewerIn their pre-registered paper [1], McCune and colleagues propose a field-based study of social versus individual learning mechanisms in an avian species (great-tailed grackles) that has been expanding its geographic range. The study forms part of a longer-term project that addresses various aspects of this species’ behaviour and biology, and the experience of the team is clear from the preprint. Assessing variation in learning mechanisms in different sections of the grackles’ distribution range, the researchers will investigate how individual learning and social transmission may impact learning about novel challenges in the environment. Considering that this is a social species, the authors expect both individual learning and social transmission to occur, when groups of grackles encounter new challenges/ opportunities in the wild. This in itself is not a very unusual idea to test [2, 3], but the authors are rigorously distinguishing between imitation, emulation, local enhancement, and social enhancement. Such rigour is certainly valuable in studies of cognition in the wild. References [1] McCune, K. B., McElreath, R., and Logan, C. J. (2019). Investigating the use of learning mechanisms in a species that is rapidly expanding its geographic range. In principle recommendation by Peer Community In Ecology. corinalogan.com/Preregistrations/g_sociallearning.html | Investigating the use of learning mechanisms in a species that is rapidly expanding its geographic range | Kelsey McCune, Richard McElreath, Corina Logan | This is one of many studies planned for our long-term research on the role of behavior and learning in rapid geographic range expansions. Project background: Behavioral flexibility, the ability to change behavior when circumstances change based on... | Behaviour & Ethology, Eco-evolutionary dynamics, Foraging, Preregistrations, Social structure, Spatial ecology, Metacommunities & Metapopulations, Zoology | Aliza le Roux | 2019-07-23 18:45:20 | View | ||
22 Nov 2021
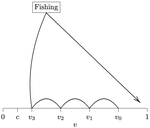
When more competitors means less harvested resourceRecommended by François Munoz based on reviews by Francois Massol, Jeremy Van Cleve and 1 anonymous reviewer based on reviews by Francois Massol, Jeremy Van Cleve and 1 anonymous reviewer
In this paper, Alan R. Rogers (2021) examines the dynamics of foraging strategies for a resource that gains value over time (e.g., ripening fruits), while there is a fixed cost of attempting to forage the resource, and once the resource is harvested nothing is left for other harvesters. For this model, not any pure foraging strategy is evolutionary stable. A mixed equilibrium exists, i.e., with a mixture of foraging strategies within the population, which is still evolutionarily unstable. Nonetheless, Alan R. Rogers shows that for a large number of competitors and/or high harvesting cost, the mixture of strategies remains close to the mixed equilibrium when simulating the dynamics. Surprisingly, in a large population individuals will less often attempt to forage the resource and will instead “go fishing”. The paper also exposes an experiment of the game with students, which resulted in a strategy distribution somehow close to the theoretical mixture of strategies. The economist John F. Nash Jr. (1950) gained the Nobel Prize of economy in 1994 for his game theoretical contributions. He gave his name to the “Nash equilibrium”, which represents a set of individual strategies that is reached whenever all the players have nothing to gain by changing their strategy while the strategies of others are unchanged. Alan R. Rogers shows that the mixed equilibrium in the foraging game is such a Nash equilibrium. Yet it is evolutionarily unstable insofar as a distribution close to the equilibrium can invade. The insights of the study are twofold. First, it sheds light on the significance of Nash equilibrium in an ecological context of foraging strategies. Second, it shows that an evolutionarily unstable state can rule the composition of the ecological system. Therefore, the contribution made by the paper should be most significant to better understand the dynamics of competitive communities and their eco-evolutionary trajectories. References Nash JF (1950) Equilibrium points in n-person games. Proceedings of the National Academy of Sciences, 36, 48–49. https://doi.org/10.1073/pnas.36.1.48 Rogers AR (2021) Beating your Neighbor to the Berry Patch. bioRxiv, 2020.11.12.380311, ver. 8 peer-reviewed and recommended by Peer Community in Ecology. https://doi.org/10.1101/2020.11.12.380311
| Beating your neighbor to the berry patch | Alan R. Rogers | <p style="text-align: justify;">Foragers often compete for resources that ripen (or otherwise improve) gradually. What strategy is optimal in this situation? It turns out that there is no optimal strategy. There is no evolutionarily stable strateg... |  | Behaviour & Ethology, Evolutionary ecology, Foraging | François Munoz | Erol Akçay, Jorge Peña, Sébastien Lion, François Rousset, Ulf Dieckmann , Troy Day , Corina Tarnita , Florence Debarre , Daniel Friedman , Vlastimil Krivan , Ulf Dieckmann | 2020-12-10 18:38:49 | View |
10 Jun 2018

A reply to “Ranging Behavior Drives Parasite Richness: A More Parsimonious Hypothesis”Charpentier MJE, Kappeler PM https://arxiv.org/abs/1805.08151v3Does elevated parasite richness in the environment affect daily path length of animals or is it the converse? An answer bringing some new elements of discussionRecommended by Cédric Sueur based on reviews by 2 anonymous reviewers based on reviews by 2 anonymous reviewers
In 2015, Brockmeyer et al. [1] suggested that mandrills (Mandrillus sphinx) may accept additional ranging costs to avoid heavily parasitized areas. Following this paper, Bicca-Marques and Calegaro-Marques [2] questioned this interpretation and presented other hypotheses. To summarize, whilst Brockmeyer et al. [1] proposed that elevated daily path length may be a consequence of elevated parasite richness, Bicca-Marques and Calegaro-Marques [2] viewed it as a cause. In this current paper, Charpentier and Kappeler [3] respond to some of the criticisms by Bicca-Marques and Calegaro-Marques and discuss the putative parsimony of the two competing scenarios. The manuscript is interesting and focuses on an important question concerning the discussion about the social organization and home range use in wild mandrills. This answer helps to move this debate forward and should stimulate more empirical studies of the role of environmentally-transmitted parasites in shaping ranging and movement patterns of wild vertebrates. Given the elements this paper brings to the topics, it should have been published in American Journal of Primatology, the journal that published the two previous articles. References [1] Brockmeyer, T., Kappeler, P. M., Willaume, E., Benoit, L., Mboumba, S., & Charpentier, M. J. E. (2015). Social organization and space use of a wild mandrill (Mandrillus sphinx) group. American Journal of Primatology, 77(10), 1036–1048. doi: 10.1002/ajp.22439 | A reply to “Ranging Behavior Drives Parasite Richness: A More Parsimonious Hypothesis” | Charpentier MJE, Kappeler PM | In a recent article, Bicca-Marques and Calegaro-Marques [2016] discussed the putative assumptions related to an interpretation we provided regarding an observed positive relationship between weekly averaged parasite richness of a group of mandrill... |  | Behaviour & Ethology, Evolutionary ecology, Foraging, Host-parasite interactions, Spatial ecology, Metacommunities & Metapopulations, Zoology | Cédric Sueur | 2018-05-22 10:59:33 | View | |
03 Apr 2020
Body temperatures, life history, and skeletal morphology in the nine-banded armadillo (Dasypus novemcinctus)Frank Knight, Cristin Connor, Ramji Venkataramanan, Robert J. Asher https://doi.org/10.17863/CAM.50971Is vertebral count in mammals influenced by developmental temperature? A study with Dasypus novemcinctusRecommended by Mar Sobral based on reviews by Darin Croft and ?Mammals show a very low level of variation in vertebral count, both among and within species, in comparison to other vertebrates [1]. Jordan’s rule for fishes states that the vertebral number among species increases with latitude, due to ambient temperatures during development [2]. Temperature has also been shown to influence vertebral count within species in fish [3], amphibians [4], and birds [5]. However, in mammals the count appears to be constrained, on the one hand, by a possible relationship between the development of the skeleton and the proliferations of cell lines with associated costs (neural malformations, cancer etc., [6]), and on the other by the cervical origin of the diaphragm [7]. References [1] Hautier L, Weisbecker V, Sánchez-Villagra MR, Goswami A, Asher RJ (2010) Skeletal development in sloths and the evolution of mammalian vertebral patterning. Proceedings of the National Academy of Sciences, 107, 18903–18908. doi: 10.1073/pnas.1010335107 | Body temperatures, life history, and skeletal morphology in the nine-banded armadillo (Dasypus novemcinctus) | Frank Knight, Cristin Connor, Ramji Venkataramanan, Robert J. Asher | <p>The nine banded armadillo (*Dasypus novemcinctus*) is the only xenarthran mammal to have naturally expanded its range into the middle latitudes of the USA. It is not known to hibernate, but has been associated with unusually labile core body te... |  | Behaviour & Ethology, Evolutionary ecology, Life history, Physiology, Zoology | Mar Sobral | 2019-11-22 22:57:31 | View | |
26 May 2023
Using repeatability of performance within and across contexts to validate measures of behavioral flexibilityMcCune KB, Blaisdell AP, Johnson-Ulrich Z, Lukas D, MacPherson M, Seitz BM, Sevchik A, Logan CJ https://doi.org/10.32942/X2R59KDo reversal learning methods measure behavioral flexibility?Recommended by Aurélie Coulon based on reviews by Maxime Dahirel and Aparajitha Ramesh based on reviews by Maxime Dahirel and Aparajitha Ramesh
Assessing the reliability of the methods we use in actually measuring the intended trait should be one of our first priorities when designing a study – especially when the trait in question is not directly observable and is measured through a proxy. This is the case for cognitive traits, which are often quantified through measures of behavioral performance. Behavioral flexibility is of particular interest in the context of great environmental changes that a lot of populations have to experiment. This type of behavioral performance is often measured through reversal learning experiments (Bond 2007). In these experiments, individuals first learn a preference, for example for an object of a certain type of form or color, associated with a reward such as food. The characteristics of the rewarded object then change, and the individuals hence have to learn these new characteristics (to get the reward). The time needed by the individual to make this change in preference has been considered a measure of behavioral flexibility. Although reversal learning experiments have been widely used, their construct validity to assess behavioral flexibility has not been thoroughly tested. This was the aim of McCune and collaborators' (2023) study, through the test of the repeatability of individual performance within and across contexts of reversal learning, in the great-tailed grackle. This manuscript presents a post-study of the preregistered study* (Logan et al. 2019) that was peer-reviewed and received an In Principle Recommendation for PCI Ecology (Coulon 2019; the initial preregistration was split into 3 post-studies).
The first hypothesis was tested by measuring the repeatability of the time needed by individuals to switch color preference in a color reversal learning task (colored tubes), over serial sessions of this task. The second one was tested by measuring the time needed by individuals to switch solutions, within 3 different contexts: (1) colored tubes, (2) plastic and (3) wooden multi-access boxes involving several ways to access food. Despite limited sample sizes, the results of these experiments suggest that there is both temporal and contextual repeatability of behavioral flexibility performance of great-tailed grackles, as measured by reversal learning experiments. Those results are a first indication of the construct validity of reversal learning experiments to assess behavioral flexibility. As highlighted by McCune and collaborators, it is now necessary to assess the discriminant validity of these experiments, i.e. checking that a different performance is obtained with tasks (experiments) that are supposed to measure different cognitive abilities. Coulon, A. (2019) Can context changes improve behavioral flexibility? Towards a better understanding of species adaptability to environmental changes. Peer Community in Ecology, 100019. https://doi.org/10.24072/pci.ecology.100019 Logan, CJ, Lukas D, Bergeron L, Folsom M, & McCune, K. (2019). Is behavioral flexibility related to foraging and social behavior in a rapidly expanding species? In Principle Acceptance by PCI Ecology of the Version on 6 Aug 2019. http://corinalogan.com/Preregistrations/g_flexmanip.html McCune KB, Blaisdell AP, Johnson-Ulrich Z, Lukas D, MacPherson M, Seitz BM, Sevchik A, Logan CJ (2023) Using repeatability of performance within and across contexts to validate measures of behavioral flexibility. EcoEvoRxiv, ver. 5 peer-reviewed and recommended by Peer Community in Ecology. https://doi.org/10.32942/X2R59K | Using repeatability of performance within and across contexts to validate measures of behavioral flexibility | McCune KB, Blaisdell AP, Johnson-Ulrich Z, Lukas D, MacPherson M, Seitz BM, Sevchik A, Logan CJ | <p style="text-align: justify;">Research into animal cognitive abilities is increasing quickly and often uses methods where behavioral performance on a task is assumed to represent variation in the underlying cognitive trait. However, because thes... | Behaviour & Ethology, Evolutionary ecology, Preregistrations, Zoology | Aurélie Coulon | 2022-08-15 20:56:42 | View | ||
15 Nov 2023

The challenges of independence: ontogeny of at-sea behaviour in a long-lived seabirdKarine Delord, Henri Weimerskirch, Christophe Barbraud https://doi.org/10.1101/2021.10.23.465439On the road to adulthood: exploring progressive changes in foraging behaviour during post-fledging immaturity using remote trackingRecommended by Blandine Doligez based on reviews by Juliet Lamb and 1 anonymous reviewerIn most vertebrate species, the period of life spanning from departure from the growing site until reaching a more advanced life stage (immature or adult) is critical. During this period, juveniles are often highly vulnerable because they have not reached the morphological, physiological and behavioural maturity levels of adults yet and are therefore at high risk of mortality, e.g. through starvation, depredation or competition (e.g. Marchetti & Price 1989, Wunderle 1991, Naef-Daenzer & Grüebler 2016). In line with this, juvenile survival is most often far lower than adult survival (e.g. Wooller et al. 1992). In species with parental care, juveniles have to acquire behavioural independence from their parents and possibly establish their own territory during this period of life. Very often, this is also the period that is least well-known in the life cycle (Cox et al. 2014, Naef-Daenzer & Grüebler 2016) because of reduced accessibility to individuals and/or adoption of low conspicuous behaviours. Therefore, our understanding of how juveniles acquire typical adult behaviours and how this progressively increases their survival prospects is still very limited (Naef-Daenzer & Grüebler 2016), and questions such as the length of this transition period or the cognitive (e.g. learning, memorization) mechanisms involved remain largely unresolved. This is particularly true regarding the acquisition of independent foraging behaviour (Marchetti & Price 1989). Because direct observations of juvenile behaviours are usually very difficult except in specific situations or at the cost of an enormous effort, the use of remote tracking devices can be particularly appealing in this context (e.g. Ponchon et al. 2013, Kays et al. 2015). Over the past decades, technical advances have allowed the monitoring of not only individuals’ movements at both large and small spatial scales but also their activities and behaviours based on different parameters recording e.g. speed of movement or diving depth (Whitford & Klimley 2019). Device miniaturization has in particular allowed smaller species to be equipped and/or longer periods of time to be monitored (e.g. Naef-Daenzer et al. 2005). This has opened up whole fields of research, and has been particularly used on marine seabirds. In these species, individuals are most often inaccessible when at sea, representing most of the time outside (and even within) the breeding season, and the life cycle of these long-lived species can include an extended immature period (up to many years) during which most of them will remain unseen, until they come back as breeders or pre-breeders (e.g. Wooller et al. 1992, Oro & Martínez-Abraín 2009). Survival has been found to increase gradually with age in these species before reaching high values characteristic of the adult stage. However, the mechanisms underlying this increase are still to be deciphered. The study by Delord et al. (2023) builds upon the hypothesis that juveniles gradually learn foraging techniques and movement strategies, improving their foraging efficiency, as previous data on flight parameters seemed to show in different long-lived bird species. Yet, these previous studies obtained data over a limited period of time, i.e. a few months at best. Whether these data could capture the whole dynamics of the progressive acquisition of foraging and movement skills can only be assessed by measuring behaviour over a longer time period and comparing it to similar data in adults, to account for seasonal variation in relation to both resource availability and energetic demands, e.g. due to molt. The present study (Delord et al. 2023) addresses these questions by taking advantage of longer-lasting recordings of the location and activity of juvenile, immature and adult birds obtained simultaneously to investigate changes over time in juvenile behaviour and thereby provide hints about how young progressively acquire foraging skills. This study is performed on Amsterdam albatrosses, a highly endangered long-lived sea bird, with obvious conservation issues (Thiebot et al. 2015). The results show progressive changes in foraging effort over the first two months after departure from the birth colony, but large differences remain between life stages over a much longer time frame. They also reveal strong variations between sexes and over time in the year. Overall, this study, therefore, confirms the need for very long-term data to be collected in order to address the question of progressive behavioural maturation and associated survival consequences in such species with strongly deferred maturity. Ideally, the same individuals should be monitored over different life stages, from the juvenile period up to adulthood, but this would require further technical development to release the issue of powering duration limitation. As reviewers emphasized in the first review round, one main challenge now remains to ascertain the outcome of the observed behavioural changes in foraging behaviour: we expect them to reflect improvement in foraging skills and thus performance of juveniles over time, but this would need to be tested. Collecting data on foraging efficiency is yet another challenge, that future technical developments may also help overcome. Importantly also, data were available only for individuals that could be caught again because the tracking device had to be retrieved from the bird. Here, a substantial fraction of the loggers (one-fifth) could not be found again (Delord et al. 2023). To what extent the birds for which no data could be obtained are a random sample of the equipped birds would also need to be assessed. The further development of remote tracking techniques allowing data to be downloaded from a long distance should help further exploration of behavioural ontogeny of juveniles while maturing and its survival consequences. Because the maturation process explored here is likely to show very different characteristics (e.g. timing and speed) in smaller / shorter-lived species (see Cox et al. 2014, Naef-Daenzer & Grüebler 2016), the development of miniaturization is also expected to allow further investigation of post-fledging behavioural maturation in a wider range of bird species. Our understanding of this crucial life phase in different types of species should thus continue to progress in the coming years. References Cox W. A., Thompson F. R. III, Cox A. S. & Faaborg J. 2014. Post-fledging survival in passerine birds and the value of post-fledging studies to conservation. Journal of Wildlife Management, 78: 183-193. https://doi.org/10.1002/jwmg.670 Delord K., Weimerskirch H. & Barbraud C. 2023. The challenges of independence: ontogeny of at-sea behaviour in a long-lived seabird. bioRxiv, ver. 6 peer-reviewed and recommended by Peer Community in Ecology. https://doi.org/10.1101/2021.10.23.465439 Kays R., Crofoot M. C., Jetz W. & Wikelski M. 2015. Terrestrial animal tracking as an eye on life and planet. Science, 348 (6240). https://doi.org/10.1126/science.aaa2478 Marchetti K: & Price T. 1989. Differences in the foraging of juvenile and adult birds: the importance of developmental constraints. Biological Reviews, 64: 51-70. https://doi.org/10.1111/j.1469-185X.1989.tb00638.x Naef-Daenzer B., Fruh D., Stalder M., Wetli P. & Weise E. 2005. Miniaturization (0.2 g) and evaluation of attachment techniques of telemetry transmitters. The Journal of Experimental Biology, 208: 4063–4068. https://doi.org/10.1242/jeb.01870 Naef-Daenzer B. & Grüebler M. U. 2016. Post-fledging survival of altricial birds: ecological determinants and adaptation. Journal of Field Ornithology, 87: 227-250. https://doi.org/10.1111/jofo.12157 Oro D. & Martínez-Abraín A. 2009. Ecology and behavior of seabirds. Marine Ecology, pp.364-389. Ponchon A., Grémillet D., Doligez B., Chambert T., Tveera T., Gonzàles-Solìs J & Boulinier T. 2013. Tracking prospecting movements involved in breeding habitat selection: insights, pitfalls and perspectives. Methods in Ecology and Evolution, 4: 143-150. https://doi.org/10.1111/j.2041-210x.2012.00259.x Thiebot J.-B., Delord K., Barbraud C., Marteau C. & Weimerskirch H. 2015. 167 individuals versus millions of hooks: bycatch mitigation in longline fisheries underlies conservation of Amsterdam albatrosses. Aquatic Conservation 26: 674-688. https://doi.org/10.1002/aqc.2578 Whitford M & Klimley A. P. An overview of behavioral, physiological, and environmental sensors used in animal biotelemetry and biologging studies. Animal Biotelemetry, 7: 26. https://doi.org/10.1186/s40317-019-0189-z Wooller R.D., Bradley J. S. & Croxall J. P. 1992. Long-term population studies of seabirds. Trends in Ecology and Evolution, 7: 111-114. https://doi.org/10.1016/0169-5347(92)90143-y Wunderle J. M. 1991. Age-specific foraging proficiency in birds. Current Ornithology, 8: 273-324. | The challenges of independence: ontogeny of at-sea behaviour in a long-lived seabird | Karine Delord, Henri Weimerskirch, Christophe Barbraud | <p style="text-align: justify;">The transition to independent foraging represents an important developmental stage in the life cycle of most vertebrate animals. Juveniles differ from adults in various life history traits and tend to survive less w... |  | Behaviour & Ethology, Foraging, Ontogeny | Blandine Doligez | 2021-10-26 07:51:49 | View | |
28 Sep 2020
The dynamics of spawning acts by a semelparous fish and its associated energetic costsCédric Tentelier, Colin Bouchard, Anaïs Bernardin, Amandine Tauzin, Jean-Christophe Aymes, Frédéric Lange, Charlotte Recapet, Jacques Rives https://doi.org/10.1101/436295Extreme weight loss: when accelerometer could reveal reproductive investment in a semelparous fish speciesRecommended by Francois-Xavier Dechaume-Moncharmont based on reviews by Aidan Jonathan Mark Hewison, Loïc Teulier and 1 anonymous reviewer based on reviews by Aidan Jonathan Mark Hewison, Loïc Teulier and 1 anonymous reviewer
Continuous observation of animal behaviour could be quite a challenge in the field, and the situation becomes even more complicated with aquatic species mostly active at night. In such cases, biologging techniques are real game changers in ecology, behavioural ecology or eco-physiology. An accelerating number of methodological applications of these tools in natural condition are thus published each year [1]. Biologging is not limited to movement ecology. For instance, fine grain information about energy expenditure can be inferred from body acceleration [2], and accelerometers has already proven useful in monitoring reproductive costs in some fish species [3,4]. The first part of the study by Tentelier et al. [5] is in line with this growing literature. It describes measurements of energy expenditure during reproduction in a fish species, Allis shad (Alosa Alosa), based on tail beat frequency and occurrence of spawning acts. The study has been convincingly conducted, and the results are important for fish biologists. But this is not the whole story: the authors added to this otherwise classical study a very original and insightful analysis which deserves closer interest. References [1] Börger L, Bijleveld AI, Fayet AL, Machovsky‐Capuska GE, Patrick SC, Street GM and Vander Wal E. (2020) Biologging special feature. J. Anim. Ecol. 89, 6–15. 10.1111/1365-2656.13163 | The dynamics of spawning acts by a semelparous fish and its associated energetic costs | Cédric Tentelier, Colin Bouchard, Anaïs Bernardin, Amandine Tauzin, Jean-Christophe Aymes, Frédéric Lange, Charlotte Recapet, Jacques Rives | <p>1. During the reproductive season, animals have to manage both their energetic budget and gamete stock. In particular, for semelparous capital breeders with determinate fecundity and no parental care other than gametic investment, the depletion... | Behaviour & Ethology, Freshwater ecology, Life history | Francois-Xavier Dechaume-Moncharmont | 2020-06-04 15:18:56 | View |
MANAGING BOARD
Julia Astegiano
Tim Coulson
Anna Eklof
Dominique Gravel
François Massol
Ben Phillips
Cyrille Violle










