Direct submissions to PCI Ecology from bioRxiv.org are possible using the B2J service
Latest recommendations

| Id | Title * | Authors * ▲ | Abstract * | Picture * | Thematic fields * | Recommender | Reviewers | Submission date | |
|---|---|---|---|---|---|---|---|---|---|
26 May 2021

Spatial distribution of local patch extinctions drives recovery dynamics in metacommunitiesCamille Saade, Sonia Kéfi, Claire Gougat-Barbera, Benjamin Rosenbaum, and Emanuel A. Fronhofer https://doi.org/10.1101/2020.12.03.409524Unity makes strength: clustered extinctions have stronger, longer-lasting effects on metacommunities dynamicsRecommended by Elodie Vercken based on reviews by David Murray-Stoker and Frederik De LaenderIn this article, Saade et al. (2021) investigate how the rate of local extinctions and their spatial distribution affect recolonization dynamics in metacommunities. They use an elegant combination of microcosm experiments with metacommunities of freshwater ciliates and mathematical modelling mirroring their experimental system. Their main findings are (i) that local patch extinctions increase both local (α-) and inter-patch (β-) diversity in a transient way during the recolonization process, (ii) that these effects depend more on the spatial distribution of extinctions (dispersed or clustered) than on their amount, and (iii) that they may spread regionally. A major strength of this study is that it highlights the importance of considering the spatial structure explicitly. Recent work on ecological networks has shown repeatedly that network structure affects the propagation of pathogens (Badham and Stocker 2010), invaders (Morel-Journel et al. 2019), or perturbation events (Gilarranz et al. 2017). Here, the spatial structure of the metacommunity is a regular grid of patches, but the distribution of extinction events may be either regularly dispersed (i.e., extinct patches are distributed evenly over the grid and are all surrounded by non-extinct patches only) or clustered (all extinct patches are neighbours). This has a direct effect on the neighbourhood of perturbed patches, and because perturbations have mostly local effects, their recovery dynamics are dominated by the composition of this immediate neighbourhood. In landscapes with dispersed extinctions, the neighbourhood of a perturbed patch is not affected by the amount of extinctions, and neither is its recovery time. In contrast, in landscapes with clustered extinctions, the amount of extinctions affects the depth of the perturbed area, which takes longer to recover when it is larger. Interestingly, the spatial distribution of extinctions here is functionally equivalent to differences in connectivity between perturbed and unperturbed patches, which results in contrasted “rescue recovery” and “mixing recovery” regimes as described by Zelnick et al. (2019).
Levins R (1969) Some Demographic and Genetic Consequences of Environmental Heterogeneity for Biological Control1. Bulletin of the Entomological Society of America, 15, 237–240. https://doi.org/10.1093/besa/15.3.237 Ruokolainen L (2013) Spatio-Temporal Environmental Correlation and Population Variability in Simple Metacommunities. PLOS ONE, 8, e72325. https://doi.org/10.1371/journal.pone.0072325 Saade C, Kefi S, Gougat-Barbera C, Rosenbaum B, Fronhofer EA (2021) Spatial distribution of local patch extinctions drives recovery dynamics in metacommunities. bioRxiv, 2020.12.03.409524, ver. 4 peer-reviewed and recommended by Peer Community in Ecology. https://doi.org/10.1101/2020.12.03.409524 | Spatial distribution of local patch extinctions drives recovery dynamics in metacommunities | Camille Saade, Sonia Kéfi, Claire Gougat-Barbera, Benjamin Rosenbaum, and Emanuel A. Fronhofer | <p style="text-align: justify;">Human activities lead more and more to the disturbance of plant and animal communities with local extinctions as a consequence. While these negative effects are clearly visible at a local scale, it is less clear how... |  | Biodiversity, Coexistence, Colonization, Community ecology, Competition, Dispersal & Migration, Experimental ecology, Landscape ecology, Spatial ecology, Metacommunities & Metapopulations | Elodie Vercken | 2020-12-08 15:55:20 | View | |
17 Mar 2021

Intra and inter-annual climatic conditions have stronger effect than grazing intensity on root growth of permanent grasslandsCatherine Picon-Cochard, Nathalie Vassal, Raphaël Martin, Damien Herfurth, Priscilla Note, Frédérique Louault https://doi.org/10.1101/2020.08.23.263137Resolving herbivore influences under climate variabilityRecommended by Jennifer Krumins based on reviews by 3 anonymous reviewersWe know that herbivory can have profound influences on plant communities with respect to their distribution and productivity (recently reviewed by Jia et al. 2018). However, the degree to which these effects are realized belowground in the rhizosphere is far less understood. Indeed, many independent studies and synthesis find that the environmental context can be more important than the direct effects of herbivore activity and its removal of plant biomass (Andriuzzi and Wall 2017, Schrama et al. 2013). In spite of dedicated attention, generalizable conclusions remain a bit elusive (Sitters and Venterink 2015). Picon-Cochard and colleagues (2021) help address this research conundrum in an elegant analysis that demonstrates the interaction between long-term cattle grazing and climatic variability on primary production aboveground and belowground. Over the course of two years, Picon-Cochard et al. (2021) measured above and belowground net primary productivity in French grasslands that had been subject to ten years of managed cattle grazing. When they compared these data with climatic trends, they find an interesting interaction among grazing intensity and climatic factors influencing plant growth. In short, and as expected, plants allocate more resources to root growth in dry years and more to above ground biomass in wet and cooler years. However, this study reveals the degree to which this is affected by cattle grazing. Grazed grasslands support warmer and dryer soils creating feedback that further and significantly promotes root growth over green biomass production. The implications of this work to understanding the capacity of grassland soils to store carbon is profound. This study addresses one brief moment in time of the long trajectory of this grazed ecosystem. The legacy of grazing does not appear to influence soil ecosystem functioning with respect to root growth except within the environmental context, in this case, climate. This supports the notion that long-term research in animal husbandry and grazing effects on landscapes is deeded. It is my hope that this study is one of many that can be used to synthesize many different data sets and build a deeper understanding of the long-term effects of grazing and herd management within the context of a changing climate. Herbivory has a profound influence upon ecosystem health and the distribution of plant communities (Speed and Austrheim 2017), global carbon storage (Chen and Frank 2020) and nutrient cycling (Sitters et al. 2020). The analysis and results presented by Picon-Cochard (2021) help to resolve the mechanisms that underly these complex effects and ultimately make projections for the future. References Andriuzzi WS, Wall DH. 2017. Responses of belowground communities to large aboveground herbivores: Meta‐analysis reveals biome‐dependent patterns and critical research gaps. Global Change Biology 23:3857-3868. doi: https://doi.org/10.1111/gcb.13675 Chen J, Frank DA. 2020. Herbivores stimulate respiration from labile and recalcitrant soil carbon pools in grasslands of Yellowstone National Park. Land Degradation & Development 31:2620-2634. doi: https://doi.org/10.1002/ldr.3656 Jia S, Wang X, Yuan Z, Lin F, Ye J, Hao Z, Luskin MS. 2018. Global signal of top-down control of terrestrial plant communities by herbivores. Proceedings of the National Academy of Sciences 115:6237-6242. doi: https://doi.org/10.1073/pnas.1707984115 Picon-Cochard C, Vassal N, Martin R, Herfurth D, Note P, Louault F. 2021. Intra and inter-annual climatic conditions have stronger effect than grazing intensity on root growth of permanent grasslands. bioRxiv, 2020.08.23.263137, version 6 peer-reviewed and recommended by PCI Ecology. doi: https://doi.org/10.1101/2020.08.23.263137 Schrama M, Veen GC, Bakker EL, Ruifrok JL, Bakker JP, Olff H. 2013. An integrated perspective to explain nitrogen mineralization in grazed ecosystems. Perspectives in Plant Ecology, Evolution and Systematics 15:32-44. doi: https://doi.org/10.1016/j.ppees.2012.12.001 Sitters J, Venterink HO. 2015. The need for a novel integrative theory on feedbacks between herbivores, plants and soil nutrient cycling. Plant and Soil 396:421-426. doi: https://doi.org/10.1007/s11104-015-2679-y Sitters J, Wubs EJ, Bakker ES, Crowther TW, Adler PB, Bagchi S, Bakker JD, Biederman L, Borer ET, Cleland EE. 2020. Nutrient availability controls the impact of mammalian herbivores on soil carbon and nitrogen pools in grasslands. Global Change Biology 26:2060-2071. doi: https://doi.org/10.1111/gcb.15023 Speed JD, Austrheim G. 2017. The importance of herbivore density and management as determinants of the distribution of rare plant species. Biological Conservation 205:77-84. doi: https://doi.org/10.1016/j.biocon.2016.11.030 | Intra and inter-annual climatic conditions have stronger effect than grazing intensity on root growth of permanent grasslands | Catherine Picon-Cochard, Nathalie Vassal, Raphaël Martin, Damien Herfurth, Priscilla Note, Frédérique Louault | <p>Background and Aims: Understanding how direct and indirect changes in climatic conditions, management, and species composition affect root production and root traits is of prime importance for the delivery of carbon sequestration services of gr... |  | Agroecology, Biodiversity, Botany, Community ecology, Ecosystem functioning | Jennifer Krumins | 2020-08-30 19:27:30 | View | |
06 Jan 2021

Comparing statistical and mechanistic models to identify the drivers of mortality within a rear-edge beech populationCathleen Petit-Cailleux, Hendrik Davi, François Lefevre, Christophe Hurson, Joseph Garrigue, Jean-André Magdalou, Elodie Magnanou and Sylvie Oddou-Muratorio https://doi.org/10.1101/645747The complexity of predicting mortality in treesRecommended by Lucía DeSoto based on reviews by Lisa Hülsmann and 2 anonymous reviewersOne of the main issues of forest ecosystems is rising tree mortality as a result of extreme weather events (Franklin et al., 1987). Eventually, tree mortality reduces forest biomass (Allen et al., 2010), although its effect on forest ecosystem fluxes seems not lasting too long (Anderegg et al., 2016). This controversy about the negative consequences of tree mortality is joined to the debate about the drivers triggering and the mechanisms accelerating tree decline. For instance, there is still room for discussion about carbon starvation or hydraulic failure determining the decay processes (Sevanto et al., 2014) or about the importance of mortality sources (Reichstein et al., 2013). Therefore, understanding and predicting tree mortality has become one of the challenges for forest ecologists in the last decade, doubling the rate of articles published on the topic (*). Although predicting the responses of ecosystems to environmental change based on the traits of species may seem a simplistic conception of ecosystem functioning (Sutherland et al., 2013), identifying those traits that are involved in the proneness of a tree to die would help to predict how forests will respond to climate threatens. (*) Number (and percentage) of articles found in Web of Sciences after searching (December the 10th, 2020) “tree mortality”: from 163 (0.006%) in 2010 to 412 (0.013%) in 2020. References Allen et al. (2010). A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. Forest ecology and management, 259(4), 660-684. doi: https://doi.org/10.1016/j.foreco.2009.09.001 | Comparing statistical and mechanistic models to identify the drivers of mortality within a rear-edge beech population | Cathleen Petit-Cailleux, Hendrik Davi, François Lefevre, Christophe Hurson, Joseph Garrigue, Jean-André Magdalou, Elodie Magnanou and Sylvie Oddou-Muratorio | <p>Since several studies have been reporting an increase in the decline of forests, a major issue in ecology is to better understand and predict tree mortality. The interactions between the different factors and the physiological processes giving ... |  | Climate change, Physiology, Population ecology | Lucía DeSoto | 2019-05-24 11:37:38 | View | |
14 Jun 2024
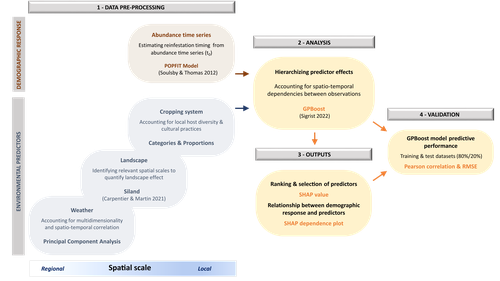
Hierarchizing multi-scale environmental effects on agricultural pest population dynamics: a case study on the annual onset of Bactrocera dorsalis population growth in Senegalese orchardsCécile Caumette, Paterne Diatta, Sylvain Piry, Marie-Pierre Chapuis, Emile Faye, Fabio Sigrist, Olivier Martin, Julien Papaïx, Thierry Brévault, Karine Berthier https://doi.org/10.1101/2023.11.10.566583Uncovering the ecology in big-data by hierarchizing multi-scale environmental effectsRecommended by Elodie Vercken based on reviews by Kévin Tougeron and Jianqiang SunAlong with the generalization of open-access practices, large, heterogeneous datasets are becoming increasingly available to ecologists (Farley et al. 2018). While such data offer exciting opportunities for unveiling original patterns and trends, they also raise new challenges regarding how to extract relevant information and actually improve our knowledge of complex ecological systems, beyond purely descriptive correlations (Dietze 2017, Farley et al. 2018). In this work, Caumette et al. (2024) develop an original ecoinformatics approach to relate multi-scale environmental factors to the temporal dynamics of a major pest in mango orchards. Their method relies on the recent tree-boosting method GPBoost (Sigrist 2022) to hierarchize the influence of environmental factors of heterogeneous nature (e.g., orchard composition and management; landscape structure; climate) on the emergence date of the oriental fruit fly, Bactrocera dorsalis. As boosting methods allows the analysis of high-dimensional data, they are particularly adapted to the exploration of such datasets, to uncover unexpected, potentially complex dependencies between ecological dynamics and multiple environmental factors (Farley et al. 2018). In this article, Caumette et al. (2024) make a special effort to guide the reader step by step through their complex analysis pipeline to make it broadly understandable to the average ecologist, which is no small feat. I particularly welcome this commitment, as making new, cutting-edge analytical methods accessible to a large community of science practitioners with varying degrees of statistical or programming expertise is a major challenge for the future of quantitative ecology. The main result of Caumette et al. (2024) is that temperature and humidity conditions both at the local and regional scales are the main predictors of B. dorsalis emergence date, while orchard management practices seem to have relatively little influence. This suggests that favourable climatic conditions may allow the persistence of small populations of B. dorsalis over the dry season, which may then act as a propagule source for early re-infestations. However, as the authors explain, the resulting regression model is not designed for predictive purposes and should not at this stage be used for decision-making in pest management. Its main interest rather resides in identifying potential key factors favoring early infestations of B. dorsalis, and help focusing future experimental field studies on the most relevant levers for integrated pest management in mango orchards. In a wider perspective, this work also provides a convincing proof-of-concept for the use of boosting methods to identify the most influential factors in large, multivariate datasets in a variety of ecological systems. It is also crucial to keep in mind that the current exponential growth in high-throughput environmental data (Lucivero 2020) could quickly come into conflict with the need to reduce the environmental footprint of research (Mariette et al. 2022). In this context, robust and accessible methods for extracting and exploiting all the information available in already existing datasets might prove essential to a sustainable pursuit of science. References Dietze MC. 2017. Ecological Forecasting. Princeton University Press Mariette J, Blanchard O, Berné O, Aumont O, Carrey J, Ligozat A-L, Lellouch E, Roche P-E, Guennebaud G, Thanwerdas J, Bardou P, Salin G, Maigne E, Servan S, Ben-Ari T 2022. An open-source tool to assess the carbon footprint of research. Environmental Research: Infrastructure and Sustainability, 2022. https://dx.doi.org/10.1088/2634-4505/ac84a4 | Hierarchizing multi-scale environmental effects on agricultural pest population dynamics: a case study on the annual onset of *Bactrocera dorsalis* population growth in Senegalese orchards | Cécile Caumette, Paterne Diatta, Sylvain Piry, Marie-Pierre Chapuis, Emile Faye, Fabio Sigrist, Olivier Martin, Julien Papaïx, Thierry Brévault, Karine Berthier | <p>Implementing integrated pest management programs to limit agricultural pest damage requires an understanding of the interactions between the environmental variability and population demographic processes. However, identifying key environmental ... |  | Demography, Landscape ecology, Statistical ecology | Elodie Vercken | 2023-12-11 17:02:08 | View | |
14 Nov 2022

Estimating abundance of a recovering transboundary brown bear population with capture-recapture modelsCécile Vanpé, Blaise Piédallu, Pierre-Yves Quenette, Jérôme Sentilles, Guillaume Queney, Santiago Palazón, Ivan Afonso Jordana, Ramón Jato, Miguel Mari Elósegui Irurtia, Jordi Solà de la Torre, Olivier Gimenez https://doi.org/10.1101/2021.12.08.471719A new and efficient approach to estimate, from protocol and opportunistic data, the size and trends of populations: the case of the Pyrenean brown bearRecommended by Nicolas BECH based on reviews by Tim Coulson, Romain Pigeault and ?In this study, the authors report a new method for estimating the abundance of the Pyrenean brown bear population. Precisely, the methodology involved aims to apply Pollock's closed robust design (PCRD) capture-recapture models to estimate population abundance and trends over time. Overall, the results encourage the use of PCRD to study populations' demographic rates, while minimizing biases due to inter-individual heterogeneity in detection probabilities. Estimating the size and trends of animal population over time is essential for informing conservation status and management decision-making (Nichols & Williams 2006). This is particularly the case when the population is small, geographically scattered, and threatened. Although several methods can be used to estimate population abundance, they may be difficult to implement when individuals are rare, elusive, solitary, largely nocturnal, highly mobile, and/or occupy large home ranges in remote and/or rugged habitats. Moreover, in such standard methods,
However, these conditions are rarely met in real populations, such as wild mammals (e.g., Bellemain et al. 2005; Solbert et al. 2006), and therefore the risk of underestimating population size can rapidly increase because the assumption of perfect detection of all individuals in the population is violated. Focusing on the critically endangered Pyrenean brown bear that was close to extinction in the mid-1990s, the study by Vanpe et al. (2022), uses protocol and opportunistic data to describe a statistical modeling exercise to construct mark-recapture histories from 2008 to 2020. Among the data, the authors collected non-invasive samples such as a mixture of hair and scat samples used for genetic identification, as well as photographic trap data of recognized individuals. These data are then analyzed in RMark to provide detection and survival estimates. The final model (i.e. PCRD capture-recapture) is then used to provide Bayesian population estimates. Results show a five-fold increase in population size between 2008 and 2020, from 13 to 66 individuals. Thus, this study represents the first published annual abundance and temporal trend estimates of the Pyrenean brown bear population since 2008. Then, although the results emphasize that the PCRD estimates were broadly close to the MRS counts and had reasonably narrow associated 95% Credibility Intervals, they also highlight that the sampling effort is different according to individuals. Indeed, as expected, the detection of an individual depends on
Overall, the PCRD capture-recapture modelling approach, involved in this study, provides robust estimates of abundance and demographic rates of the Pyrenean brown bear population (with associated uncertainty) while minimizing and considering bias due to inter-individual heterogeneity in detection probabilities. The authors conclude that mark-recapture provides useful population estimates and urge wildlife ecologists and managers to use robust approaches, such as the RDPC capture-recapture model, when studying large mammal populations. This information is essential to inform management decisions and assess the conservation status of populations.
References Bellemain, E.V.A., Swenson, J.E., Tallmon, D., Brunberg, S. and Taberlet, P. (2005). Estimating population size of elusive animals with DNA from hunter-collected feces: four methods for brown bears. Cons. Biol. 19(1), 150-161. https://doi.org/10.1111/j.1523-1739.2005.00549.x Nichols, J.D. and Williams, B.K. (2006). Monitoring for conservation. Trends Ecol. Evol. 21(12), 668-673. https://doi.org/10.1016/j.tree.2006.08.007 Otis, D.L., Burnham, K.P., White, G.C. and Anderson, D.R. (1978). Statistical inference from capture data on closed animal populations. Wildlife Monographs (62), 3-135. Solberg, K.H., Bellemain, E., Drageset, O.M., Taberlet, P. and Swenson, J.E. (2006). An evaluation of field and non-invasive genetic methods to estimate brown bear (Ursus arctos) population size. Biol. Conserv. 128(2), 158-168. https://doi.org/10.1016/j.biocon.2005.09.025 Vanpé C, Piédallu B, Quenette P-Y, Sentilles J, Queney G, Palazón S, Jordana IA, Jato R, Elósegui Irurtia MM, de la Torre JS, and Gimenez O (2022) Estimating abundance of a recovering transboundary brown bear population with capture-recapture models. bioRxiv, 2021.12.08.471719, ver. 4 recommended and peer-reviewed by PCI Ecology. https://doi.org/10.1101/2021.12.08.471719 | Estimating abundance of a recovering transboundary brown bear population with capture-recapture models | Cécile Vanpé, Blaise Piédallu, Pierre-Yves Quenette, Jérôme Sentilles, Guillaume Queney, Santiago Palazón, Ivan Afonso Jordana, Ramón Jato, Miguel Mari Elósegui Irurtia, Jordi Solà de la Torre, Olivier Gimenez | <p>Estimating the size of small populations of large mammals can be achieved via censuses, or complete counts, of recognizable individuals detected over a time period: minimum detected (population) size (MDS). However, as a population grows larger... |  | Conservation biology, Demography, Population ecology | Nicolas BECH | 2022-01-20 10:49:59 | View | |
12 Jun 2019
Environmental heterogeneity drives tsetse fly population dynamics and controlCecilia H, Arnoux S, Picault S, Dicko A, Seck MT, Sall B, Bassene M, Vreysen M, Pagabeleguem S, Bance A, Bouyer J, Ezanno P https://doi.org/10.1101/493650Modeling jointly landscape complexity and environmental heterogeneity to envision new strategies for tsetse flies controlRecommended by Benjamin Roche based on reviews by Timothée Vergne and 1 anonymous reviewerToday, understanding spatio-temporal dynamics of pathogens is pivotal to understand their transmission and controlling them. First, understanding this dynamics can reveal the ecology of their transmission [1]. Indeed, such knowledge, based on data that are quite easy to access, can shed light on transmission modes, which could rely on different animal species that can be spatially distributed in a non-uniform way [2]. This is especially true for pathogens with complex life-cycles, despite that investigating such dynamics is very challenging and rely mostly on mathematical models. References [1] Grenfell, B. T., Bjørnstad, O. N., & Kappey, J. (2001). Travelling waves and spatial hierarchies in measles epidemics. Nature, 414(6865), 716-723. doi: 10.1038/414716a | Environmental heterogeneity drives tsetse fly population dynamics and control | Cecilia H, Arnoux S, Picault S, Dicko A, Seck MT, Sall B, Bassene M, Vreysen M, Pagabeleguem S, Bance A, Bouyer J, Ezanno P | <p>A spatially and temporally heterogeneous environment may lead to unexpected population dynamics. Knowledge still is needed on which of the local environment properties favour population maintenance at larger scale. For pathogen vectors, such as... |  | Biological control, Population ecology, Spatial ecology, Metacommunities & Metapopulations | Benjamin Roche | 2018-12-14 12:13:39 | View | |
03 Jan 2024
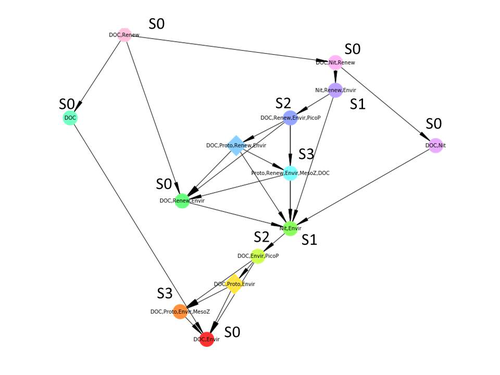
Diagnosis of planktonic trophic network dynamics with sharp qualitative changesCedric Gaucherel, Stolian Fayolle, Raphael Savelli, Olivier Philippine, Franck Pommereau, Christine Dupuy https://doi.org/10.1101/2023.06.29.547055A new approach to describe qualitative changes of complex trophic networksRecommended by Francis Raoul based on reviews by Tim Coulson and 1 anonymous reviewerModelling the temporal dynamics of trophic networks has been a key challenge for community ecologists for decades, especially when anthropogenic and natural forces drive changes in species composition, abundance, and interactions over time. So far, most modelling methods fail to incorporate the inherent complexity of such systems, and its variability, to adequately describe and predict temporal changes in the topology of trophic networks. Taking benefit from theoretical computer science advances, Gaucherel and colleagues (2024) propose a new methodological framework to tackle this challenge based on discrete-event Petri net methodology. To introduce the concept to naïve readers the authors provide a useful example using a simplistic predator-prey model. The core biological system of the article is a freshwater trophic network of western France in the Charente-Maritime marshes of the French Atlantic coast. A directed graph describing this system was constructed to incorporate different functional groups (phytoplankton, zooplankton, resources, microbes, and abiotic components of the environment) and their interactions. Rules and constraints were then defined to, respectively, represent physiochemical, biological, or ecological processes linking network components, and prevent the model from simulating unrealistic trajectories. Then the full range of possible trajectories of this mechanistic and qualitative model was computed. The model performed well enough to successfully predict a theoretical trajectory plus two trajectories of the trophic network observed in the field at two different stations, therefore validating the new methodology introduced here. The authors conclude their paper by presenting the power and drawbacks of such a new approach to qualitatively model trophic networks dynamics. Reference Cedric Gaucherel, Stolian Fayolle, Raphael Savelli, Olivier Philippine, Franck Pommereau, Christine Dupuy (2024) Diagnosis of planktonic trophic network dynamics with sharp qualitative changes. bioRxiv 2023.06.29.547055, ver. 2 peer-reviewed and recommended by Peer Community in Ecology. https://doi.org/10.1101/2023.06.29.547055 | Diagnosis of planktonic trophic network dynamics with sharp qualitative changes | Cedric Gaucherel, Stolian Fayolle, Raphael Savelli, Olivier Philippine, Franck Pommereau, Christine Dupuy | <p>Trophic interaction networks are notoriously difficult to understand and to diagnose (i.e., to identify contrasted network functioning regimes). Such ecological networks have many direct and indirect connections between species, and these conne... |  | Community ecology, Ecosystem functioning, Food webs, Freshwater ecology, Interaction networks, Microbial ecology & microbiology | Francis Raoul | Tim Coulson | 2023-07-03 10:42:34 | View |
11 Oct 2023

Identification of microbial exopolymer producers in sandy and muddy intertidal sediments by compound-specific isotope analysisCédric Hubas, Julie Gaubert-Boussarie, An-Sofie D’Hondt, Bruno Jesus, Dominique Lamy, Vona Meleder, Antoine Prins, Philippe Rosa, Willem Stock, Koen Sabbe https://doi.org/10.1101/2022.12.02.516908Disentangling microbial exopolymer dynamics in intertidal sedimentsRecommended by Ute Risse-Buhl and Nils Rädecker based on reviews by 2 anonymous reviewers based on reviews by 2 anonymous reviewers
The secretion of extracellular polymeric substances (EPS) enables microorganisms to shape and interact with their environment [1]. EPS support cell adhesion and motility, offer protection from unfavorable conditions, and facilitate nutrient acquisition and transfer between microorganisms [2]. EPS production and consumption thus control the formation and structural organization of biofilms [3]. However, in marine environments, our understanding of the sources and composition of EPS is limited. References
| Identification of microbial exopolymer producers in sandy and muddy intertidal sediments by compound-specific isotope analysis | Cédric Hubas, Julie Gaubert-Boussarie, An-Sofie D’Hondt, Bruno Jesus, Dominique Lamy, Vona Meleder, Antoine Prins, Philippe Rosa, Willem Stock, Koen Sabbe | <p style="text-align: justify;">Extracellular polymeric substances (EPS) refer to a wide variety of high molecular weight molecules secreted outside the cell membrane by biofilm microorganisms. In the present study, EPS from marine microphytobenth... |  | Biodiversity, Ecological stoichiometry, Ecosystem functioning, Food webs, Marine ecology, Microbial ecology & microbiology, Soil ecology | Ute Risse-Buhl | 2022-12-06 14:13:11 | View | |
28 Sep 2020
The dynamics of spawning acts by a semelparous fish and its associated energetic costsCédric Tentelier, Colin Bouchard, Anaïs Bernardin, Amandine Tauzin, Jean-Christophe Aymes, Frédéric Lange, Charlotte Recapet, Jacques Rives https://doi.org/10.1101/436295Extreme weight loss: when accelerometer could reveal reproductive investment in a semelparous fish speciesRecommended by Francois-Xavier Dechaume-Moncharmont based on reviews by Aidan Jonathan Mark Hewison, Loïc Teulier and 1 anonymous reviewer based on reviews by Aidan Jonathan Mark Hewison, Loïc Teulier and 1 anonymous reviewer
Continuous observation of animal behaviour could be quite a challenge in the field, and the situation becomes even more complicated with aquatic species mostly active at night. In such cases, biologging techniques are real game changers in ecology, behavioural ecology or eco-physiology. An accelerating number of methodological applications of these tools in natural condition are thus published each year [1]. Biologging is not limited to movement ecology. For instance, fine grain information about energy expenditure can be inferred from body acceleration [2], and accelerometers has already proven useful in monitoring reproductive costs in some fish species [3,4]. The first part of the study by Tentelier et al. [5] is in line with this growing literature. It describes measurements of energy expenditure during reproduction in a fish species, Allis shad (Alosa Alosa), based on tail beat frequency and occurrence of spawning acts. The study has been convincingly conducted, and the results are important for fish biologists. But this is not the whole story: the authors added to this otherwise classical study a very original and insightful analysis which deserves closer interest. References [1] Börger L, Bijleveld AI, Fayet AL, Machovsky‐Capuska GE, Patrick SC, Street GM and Vander Wal E. (2020) Biologging special feature. J. Anim. Ecol. 89, 6–15. 10.1111/1365-2656.13163 | The dynamics of spawning acts by a semelparous fish and its associated energetic costs | Cédric Tentelier, Colin Bouchard, Anaïs Bernardin, Amandine Tauzin, Jean-Christophe Aymes, Frédéric Lange, Charlotte Recapet, Jacques Rives | <p>1. During the reproductive season, animals have to manage both their energetic budget and gamete stock. In particular, for semelparous capital breeders with determinate fecundity and no parental care other than gametic investment, the depletion... | Behaviour & Ethology, Freshwater ecology, Life history | Francois-Xavier Dechaume-Moncharmont | 2020-06-04 15:18:56 | View | ||
19 Dec 2020
Hough transform implementation to evaluate the morphological variability of the moon jellyfish (Aurelia spp.)Céline Lacaux, Agnès Desolneux, Justine Gadreaud, Bertrand Martin-Garin and Alain Thiéry https://doi.org/10.1101/2020.03.11.986984A new member of the morphometrics jungle to better monitor vulnerable lagoonsRecommended by Vincent Bonhomme based on reviews by Julien Claude and 1 anonymous reviewerIn the recent years, morphometrics, the quantitative description of shape and its covariation [1] gained considerable momentum in evolutionary ecology. Using the form of organisms to describe, classify and try to understand their diversity can be traced back at least to Aristotle. More recently, two successive revolutions rejuvenated this idea [1–3]: first, a proper mathematical refoundation of the theory of shape, then a technical revolution in the apparatus able to acquire raw data. By using a feature extraction method and planning its massive use on data acquired by aerial drones, the study by Lacaux and colleagues [4] retraces this curse of events. The sample sizes studied here were too low to allow finer-grained ecophysiological investigations. That being said, the proof-of-concept is convincing and this paper paths the way for an operational and innovative approach to the ecological monitoring of sensible aquatic ecosystems. References [1] Kendall, D. G. (1989). A survey of the statistical theory of shape. Statistical Science, 87-99. doi: https://doi.org/10.1214/ss/1177012589 | Hough transform implementation to evaluate the morphological variability of the moon jellyfish (Aurelia spp.) | Céline Lacaux, Agnès Desolneux, Justine Gadreaud, Bertrand Martin-Garin and Alain Thiéry | <p>Variations of the animal body plan morphology and morphometry can be used as prognostic tools of their habitat quality. The potential of the moon jellyfish (Aurelia spp.) as a new model organism has been poorly tested. However, as a tetramerous... |  | Morphometrics | Vincent Bonhomme | 2020-03-18 17:40:51 | View |
MANAGING BOARD
Julia Astegiano
Tim Coulson
Anna Eklof
Dominique Gravel
François Massol
Ben Phillips
Cyrille Violle










