Latest recommendations

| Id | Title | Authors | Abstract | Picture | Thematic fields | Recommender | Reviewers▼ | Submission date | |
|---|---|---|---|---|---|---|---|---|---|
01 Feb 2020

Touchy matter: the delicate balance between Morgan’s canon and open-minded description of advanced cognitive skills in the animalRecommended by Francois-Xavier Dechaume-Moncharmont based on reviews by Valérie Dufour and Alex Taylor based on reviews by Valérie Dufour and Alex Taylor
In a recent paper published in PNAS, Fayet et al. [1] reported scarce field observations of two Atlantic puffins (four years apart) apparently scratching their bodies using sticks, which was interpreted by the authors as evidence of tool use in this species. In a short response, Benjamin Farrar [2] raises serious concerns about this interpretation and proposes simpler, more parsimonious, mechanisms explaining the observed behaviour: a textbook case of Morgan's canon. References [1] Fayet, A. L., Hansen, E. S., and Biro, D. (2020). Evidence of tool use in a seabird. Proceedings of the National Academy of Sciences, 117(3), 1277–1279. doi: 10.1073/pnas.1918060117 | Evidence of tool use in a seabird? | Benjamin G. Farrar | Fayet, Hansen and Biro (1) provide two observations of Atlantic puffins, *Fratercula arctica*, performing self-directed actions while holding a stick in their beaks. The authors interpret this as evidence of tool use as they suggest that the stick... |  | Behaviour & Ethology | Francois-Xavier Dechaume-Moncharmont | 2020-01-22 11:55:27 | View | |
25 May 2021

Clumpy coexistence in phytoplankton: The role of functional similarity in community assemblyCaio Graco-Roza, Angel M. Segura, Carla Kruk, Patricia Domingos, Janne Soininen, Marcelo M. Marinho https://doi.org/10.1101/869966Environmental heterogeneity drives phytoplankton community assembly patterns in a tropical riverine systemRecommended by Cédric Hubas and Eric Goberville and Eric Goberville based on reviews by Eric Goberville and Dominique Lamy based on reviews by Eric Goberville and Dominique Lamy
What predisposes two individuals to form and maintain a relationship is a fundamental question. Using facial recognition to see whether couples' faces change over time to become more and more similar, psychology researchers have concluded that couples tend to be formed from the start between people whose faces are more similar than average [1]. As the saying goes, birds of a feather flock together. And what about in nature? Are these rules of assembly valid for communities of different species? In his seminal contribution, Robert MacArthur (1984) wrote ‘To do science is to search for repeated patterns’ [2]. Identifying the mechanisms that govern the arrangement of life is a hot research topic in the field of ecology for decades, and an absolutely essential prerequisite to answer the outstanding question of what shape ecological patterns in multi-species communities such as species-area relationships, relative species abundances, or spatial and temporal turnover of community composition; amid others [3]. To explain ecological patterns in nature, some rely on the concept that every species - through evolutionary processes and the acquisition of a unique set of traits that allow a species to be adapted to its abiotic and biotic environment - occupies a unique niche: Species coexistence comes as the result of niche differentiation [4,5]. Such a view has been challenged by the recognition of the key role of neutral processes [6], however, in which demographic stochasticity contributes to shape multi-species communities and to explain why congener species coexist much more frequently than expected by chance [7,8]. While the niche-based and neutral theories appear seemingly opposed at first sight [9], the dichotomy may be more philosophical than empirical [4,5]. Many examples have come to support that both concepts are not incompatible as they together influence the structure, diversity and functioning of communities [10], and are simply extreme cases of a continuum [11]. From this perspective, extrinsic factors, i.e., environmental heterogeneity, may influence the location of a given community along the niche-neutrality continuum. The walk of species in nature is therefore neither random nor ecologically predestined. In microbial assemblages, the co-existence of these two antagonistic mechanisms has been shown both theoretically and empirically. It has been shown that a combination of stabilising (niche) and equalising (neutral) mechanisms was responsible for the existence of groups of coexistent species (clumps) in a phytoplankton rich community [12]. Analysing interannual changes (2003-2009) in the weekly abundance of diatoms and dinoflagellates located in a temperate coastal ecosystem of the Western English Channel, Mutshinda et al. [13] found a mixture of biomass dynamics consistent with the neutrality-niche continuum hypothesis. While niche processes explained the dynamic of phytoplankton functional groups (i.e., diatoms vs. dinoflagellates) in terms of biomass, neutral processes mainly dominated - 50 to 75% of the time - the dynamics at the species level within functional groups [13]. From one endpoint to another, defining the location of a community along the continuum is all matter of scale [4,11]. In their study, testing predictions made by an emergent neutrality model, Graco-Roza et al. [14] provide empirical evidence that neutral and niche processes joined together to shape and drive planktonic communities in a riverine ecosystem. Body size - the 'master trait' - is used here as a discriminant ecological dimension along the niche axis. From their analysis, they not only show that the specific abundance is organised in clumps and gaps along the niche axis, but also reveal that different clumps exist along the river course. They identify two main clumps in body size - with species belonging to three different morphologically-based functional groups - and characterise that among-species differences in biovolume are driven by functional redundancy at the clump level; species functional distinctiveness being related to the relative biovolume of species. By grouping their variables according to seasons (cold-dry vs. warm-wet) or river elevation profile (upper, medium and lower course), they hereby highlight how environmental heterogeneity contributes to shape species assemblages and their dynamics and conclude that emergent neutrality models are a powerful approach to explain species coexistence; and therefore ecological patterns. References [1] Tea-makorn PP, Kosinski M (2020) Spouses’ faces are similar but do not become more similar with time. Scientific Reports, 10, 17001. https://doi.org/10.1038/s41598-020-73971-8. [2] MacArthur RH (1984) Geographical Ecology: Patterns in the Distribution of Species. Princeton University Press. [3] Vellend M (2020) The Theory of Ecological Communities (MPB-57). Princeton University Press. [4] Wennekes PL, Rosindell J, Etienne RS (2012) The Neutral—Niche Debate: A Philosophical Perspective. Acta Biotheoretica, 60, 257–271. https://doi.org/10.1007/s10441-012-9144-6. [5] Gravel D, Guichard F, Hochberg ME (2011) Species coexistence in a variable world. Ecology Letters, 14, 828–839. https://doi.org/10.1111/j.1461-0248.2011.01643.x. [6] Hubbell SP (2001) The Unified Neutral Theory of Biodiversity and Biogeography (MPB-32). Princeton University Press. [7] Leibold MA, McPeek MA (2006) Coexistence of the Niche and Neutral Perspectives in Community Ecology. Ecology, 87, 1399–1410. https://doi.org/10.1890/0012-9658(2006)87[1399:COTNAN]2.0.CO;2. [8] Pielou EC (1977) The Latitudinal Spans of Seaweed Species and Their Patterns of Overlap. Journal of Biogeography, 4, 299–311. https://doi.org/10.2307/3038189. [9] Holt RD (2006) Emergent neutrality. Trends in Ecology & Evolution, 21, 531–533. https://doi.org/10.1016/j.tree.2006.08.003. [10] Scheffer M, Nes EH van (2006) Self-organized similarity, the evolutionary emergence of groups of similar species. Proceedings of the National Academy of Sciences, 103, 6230–6235. https://doi.org/10.1073/pnas.0508024103. [11] Gravel D, Canham CD, Beaudet M, Messier C (2006) Reconciling niche and neutrality: the continuum hypothesis. Ecology Letters, 9, 399–409. https://doi.org/10.1111/j.1461-0248.2006.00884.x. [12] Vergnon R, Dulvy NK, Freckleton RP (2009) Niches versus neutrality: uncovering the drivers of diversity in a species-rich community. Ecology Letters, 12, 1079–1090. https://doi.org/10.1111/j.1461-0248.2009.01364.x. [13] Mutshinda CM, Finkel ZV, Widdicombe CE, Irwin AJ (2016) Ecological equivalence of species within phytoplankton functional groups. Functional Ecology, 30, 1714–1722. https://doi.org/10.1111/1365-2435.12641. [14] Graco-Roza C, Segura AM, Kruk C, Domingos P, Soininen J, Marinho MM (2021) Clumpy coexistence in phytoplankton: The role of functional similarity in community assembly. bioRxiv, 869966, ver. 6 peer-reviewed and recommended by Peer Community in Ecology. https://doi.org/10.1101/869966
| Clumpy coexistence in phytoplankton: The role of functional similarity in community assembly | Caio Graco-Roza, Angel M. Segura, Carla Kruk, Patricia Domingos, Janne Soininen, Marcelo M. Marinho | <p style="text-align: justify;">Emergent neutrality (EN) suggests that species must be sufficiently similar or sufficiently different in their niches to avoid interspecific competition. Such a scenario results in a transient pattern with clumps an... |  | Coexistence, Community ecology, Theoretical ecology | Cédric Hubas | 2020-01-23 16:11:32 | View | |
21 Nov 2023
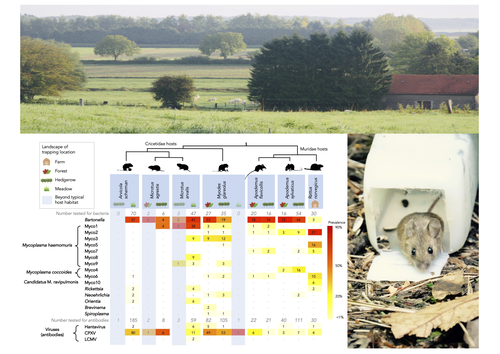
Pathogen community composition and co-infection patterns in a wild community of rodentsJessica Lee Abbate, Maxime Galan, Maria Razzauti, Tarja Sironen, Liina Voutilainen, Heikki Henttonen, Patrick Gasqui, Jean-François Cosson, Nathalie Charbonnel https://doi.org/10.1101/2020.02.09.940494Reservoirs of pestilence: what pathogen and rodent community analyses can tell us about transmission riskRecommended by Francois Massol based on reviews by Adrian Diaz, Romain Pigeault and 1 anonymous reviewer based on reviews by Adrian Diaz, Romain Pigeault and 1 anonymous reviewer
Rodents are well known as one of the main animal groups responsible for human-transmitted pathogens. As such, it seems logical to try and survey what kinds of pathogenic microbes might be harboured by wild rodents, in order to establish some baseline surveillance and prevent future zoonotic outbreaks (Bernstein et al., 2022). This is exactly what Abbate et al. (2023) endeavoured and their findings are intimidating. Based on quite a large sampling effort, they collected more than 700 rodents of seven species around two villages in northeastern France. They looked for molecular markers indicative of viral and bacterial infections and proceeded to analyze their pathogen communities using multivariate techniques. Variation in the prevalence of the different pathogens was found among host species, with e.g. signs of CPXV more prevalent in Cricetidae while some Mycoplasma strains were more prevalent in Muridae. Co-circulation of pathogens was found in all species, with some evidencing signs of up to 12 different pathogen taxa. The diversity of co-circulating pathogens was markedly different between host species and higher in adult hosts, but not affected by sex. The dataset also evinced some slight differences between habitats, with meadows harbouring a little more diversity of rodent pathogens than forests. Less intuitively, some pathogen associations seemed quite repeatable, such as the positive association of Bartonella spp. with CPXV in the montane water vole. The study allowed the authors to test several associations already described in the literature, including associations between different hemotropic Mycoplasma species. I strongly invite colleagues interested in zoonoses, emerging pandemics and more generally One Health to read the paper of Abbate et al. (2023) and try to replicate them across the world. To prevent the next sanitary crises, monitoring rodents, and more generally vertebrates, population demographics is a necessary and enlightening step (Johnson et al., 2020), but insufficient. Following the lead of colleagues working on rodent ectoparasites (Krasnov et al., 2014), we need more surveys like the one described by Abbate et al. (2023) to understand the importance of the dilution effect in the prevalence and transmission of microbial pathogens (Andreazzi et al., 2023) and the formation of epidemics. We also need other similar studies to assess the potential of different rodent species to carry pathogens more or less capable of infecting other mammalian species (Morand et al., 2015), in other places in the world. References Abbate, J. L., Galan, M., Razzauti, M., Sironen, T., Voutilainen, L., Henttonen, H., Gasqui, P., Cosson, J.-F. & Charbonnel, N. (2023) Pathogen community composition and co-infection patterns in a wild community of rodents. BioRxiv, ver.4 peer-reviewed and recommended by Peer Community in Ecology. https://doi.org/10.1101/2020.02.09.940494 Andreazzi, C. S., Martinez-Vaquero, L. A., Winck, G. R., Cardoso, T. S., Teixeira, B. R., Xavier, S. C. C., Gentile, R., Jansen, A. M. & D'Andrea, P. S. (2023) Vegetation cover and biodiversity reduce parasite infection in wild hosts across ecological levels and scales. Ecography, 2023, e06579. | Pathogen community composition and co-infection patterns in a wild community of rodents | Jessica Lee Abbate, Maxime Galan, Maria Razzauti, Tarja Sironen, Liina Voutilainen, Heikki Henttonen, Patrick Gasqui, Jean-François Cosson, Nathalie Charbonnel | <p style="text-align: justify;">Rodents are major reservoirs of pathogens that can cause disease in humans and livestock. It is therefore important to know what pathogens naturally circulate in rodent populations, and to understand the factors tha... |  | Biodiversity, Coexistence, Community ecology, Eco-immunology & Immunity, Epidemiology, Host-parasite interactions, Population ecology, Species distributions | Francois Massol | 2020-02-11 12:42:28 | View | |
19 Dec 2020
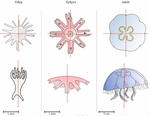
Hough transform implementation to evaluate the morphological variability of the moon jellyfish (Aurelia spp.)Céline Lacaux, Agnès Desolneux, Justine Gadreaud, Bertrand Martin-Garin and Alain Thiéry https://doi.org/10.1101/2020.03.11.986984A new member of the morphometrics jungle to better monitor vulnerable lagoonsRecommended by Vincent Bonhomme based on reviews by Julien Claude and 1 anonymous reviewerIn the recent years, morphometrics, the quantitative description of shape and its covariation [1] gained considerable momentum in evolutionary ecology. Using the form of organisms to describe, classify and try to understand their diversity can be traced back at least to Aristotle. More recently, two successive revolutions rejuvenated this idea [1–3]: first, a proper mathematical refoundation of the theory of shape, then a technical revolution in the apparatus able to acquire raw data. By using a feature extraction method and planning its massive use on data acquired by aerial drones, the study by Lacaux and colleagues [4] retraces this curse of events. The sample sizes studied here were too low to allow finer-grained ecophysiological investigations. That being said, the proof-of-concept is convincing and this paper paths the way for an operational and innovative approach to the ecological monitoring of sensible aquatic ecosystems. References [1] Kendall, D. G. (1989). A survey of the statistical theory of shape. Statistical Science, 87-99. doi: https://doi.org/10.1214/ss/1177012589 | Hough transform implementation to evaluate the morphological variability of the moon jellyfish (Aurelia spp.) | Céline Lacaux, Agnès Desolneux, Justine Gadreaud, Bertrand Martin-Garin and Alain Thiéry | <p>Variations of the animal body plan morphology and morphometry can be used as prognostic tools of their habitat quality. The potential of the moon jellyfish (Aurelia spp.) as a new model organism has been poorly tested. However, as a tetramerous... |  | Morphometrics | Vincent Bonhomme | 2020-03-18 17:40:51 | View | |
11 Mar 2021
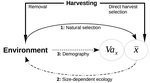
Size-dependent eco-evolutionary feedbacks in fisheriesEric Edeline and Nicolas Loeuille https://doi.org/10.1101/2020.04.03.022905“Hidden” natural selection and the evolution of body size in harvested stocksRecommended by Simon Blanchet based on reviews by Jean-François Arnoldi and 1 anonymous reviewerHumans are exploiting biological resources since thousands of years. Exploitation of biological resources has become particularly intense since the beginning of the 20th century and the steep increase in the worldwide human population size. Marine and freshwater fishes are not exception to that rule, and they have been (and continue to be) strongly harvested as a source of proteins for humans. For some species, fishery has been so intense that natural stocks have virtually collapsed in only a few decades. The worst example begin that of the Northwest Atlantic cod that has declined by more than 95% of its historical biomasses in only 20-30 years of intensive exploitation (Frank et al. 2005). These rapid and steep changes in biomasses have huge impacts on the entire ecosystems since species targeted by fisheries are often at the top of trophic chains (Frank et al. 2005). Beyond demographic impacts, fisheries also have evolutionary impacts on populations, which can also indirectly alter ecosystems (Uusi-Heikkilä et al. 2015; Palkovacs et al. 2018). Fishermen generally focus on the largest specimens, and hence exert a strong selective pressure against these largest fish (which is called “harvest selection”). There is now ample evidence that harvest selection can lead to rapid evolutionary changes in natural populations toward small individuals (Kuparinen & Festa-Bianchet 2017). These evolutionary changes are of course undesirable from a human perspective, and have attracted many scientific questions. Nonetheless, the consequence of harvest selection is not always observable in natural populations, and there are cases in which no phenotypic change (or on the contrary an increase in mean body size) has been observed after intense harvest pressures. In a conceptual Essay, Edeline and Loeuille (Edeline & Loeuille 2020) propose novel ideas to explain why the evolutionary consequences of harvest selection can be so diverse, and how a cross talk between ecological and evolutionary dynamics can explain patterns observed in natural stocks. The general and novel concept proposed by Edeline and Loeuille is actually as old as Darwin’s book; The Origin of Species (Darwin 1859). It is based on the simple idea that natural selection acting on harvested populations can actually be strong, and counter-balance (or on the contrary reinforce) the evolutionary consequence of harvest selection. Although simple, the idea that natural and harvest selection are jointly shaping contemporary evolution of exploited populations lead to various and sometimes complex scenarios that can (i) explain unresolved empirical patterns and (ii) refine predictions regarding the long-term viability of exploited populations. The Edeline and Loeuille’s crafty inspiration is that natural selection acting on exploited populations is itself an indirect consequence of harvest (Edeline & Loeuille 2020). They suggest that, by modifying the size structure of populations (a key parameter for ecological interactions), harvest indirectly alters interactions between populations and their biotic environment through competition and predation, which changes the ecological theatre and hence the selective pressures acting back to populations. They named this process “size-dependent eco-evolutionary feedback loops” and develop several scenarios in which these feedback loops ultimately deviate the evolutionary outcome of harvest selection from expectation. The scenarios they explore are based on strong theoretical knowledge, and range from simple ones in which a single species (the harvest species) is evolving to more complex (and realistic) ones in which multiple (e.g. the harvest species and its prey) species are co-evolving. I will not come into the details of each scenario here, and I will let the readers (re-)discovering the complex beauty of biological life and natural selection. Nonetheless, I will emphasize the importance of considering these eco-evolutionary processes altogether to fully grasp the response of exploited populations. Edeline and Loeuille convincingly demonstrate that reduced body size due to harvest selection is obviously not the only response of exploited fish populations when natural selection is jointly considered (Edeline & Loeuille 2020). On the contrary, they show that –under some realistic ecological circumstances relaxing exploitative competition due to reduced population densities- natural selection can act antagonistically, and hence favour stable body size in exploited populations. Although this seems further desirable from a human perspective than a downsizing of exploited populations, it is actually mere window dressing as Edeline and Loeuille further showed that this response is accompanied by an erosion of the evolvability –and hence a lowest probability of long-term persistence- of these exploited populations. Humans, by exploiting biological resources, are breaking the relative equilibrium of complex entities, and the response of populations to this disturbance is itself often complex and heterogeneous. In this Essay, Edeline and Loeuille provide –under simple terms- the theoretical and conceptual bases required to improve predictions regarding the evolutionary responses of natural populations to exploitation by humans (Edeline & Loeuille 2020). An important next step will be to generate data and methods allowing confronting the empirical reality to these novel concepts (e.g. (Monk et al. 2021), so as to identify the most likely evolutionary scenarios sustaining biological responses of exploited populations, and hence to set the best management plans for the long-term sustainability of these populations. References Darwin, C. (1859). On the Origin of Species by Means of Natural Selection. John Murray, London. Edeline, E. & Loeuille, N. (2021) Size-dependent eco-evolutionary feedbacks in fisheries. bioRxiv, 2020.04.03.022905, ver. 4 peer-reviewed and recommended by PCI Ecology. doi: https://doi.org/10.1101/2020.04.03.022905 Frank, K.T., Petrie, B., Choi, J. S. & Leggett, W.C. (2005). Trophic Cascades in a Formerly Cod-Dominated Ecosystem. Science, 308, 1621–1623. doi: https://doi.org/10.1126/science.1113075 Kuparinen, A. & Festa-Bianchet, M. (2017). Harvest-induced evolution: insights from aquatic and terrestrial systems. Philos. Trans. R. Soc. B Biol. Sci., 372, 20160036. doi: https://doi.org/10.1098/rstb.2016.0036 Monk, C.T., Bekkevold, D., Klefoth, T., Pagel, T., Palmer, M. & Arlinghaus, R. (2021). The battle between harvest and natural selection creates small and shy fish. Proc. Natl. Acad. Sci., 118, e2009451118. doi: https://doi.org/10.1073/pnas.2009451118 Palkovacs, E.P., Moritsch, M.M., Contolini, G.M. & Pelletier, F. (2018). Ecology of harvest-driven trait changes and implications for ecosystem management. Front. Ecol. Environ., 16, 20–28. doi: https://doi.org/10.1002/fee.1743 Uusi-Heikkilä, S., Whiteley, A.R., Kuparinen, A., Matsumura, S., Venturelli, P.A., Wolter, C., et al. (2015). The evolutionary legacy of size-selective harvesting extends from genes to populations. Evol. Appl., 8, 597–620. doi: https://doi.org/10.1111/eva.12268 | Size-dependent eco-evolutionary feedbacks in fisheries | Eric Edeline and Nicolas Loeuille | <p>Harvesting may drive body downsizing along with population declines and decreased harvesting yields. These changes are commonly construed as direct consequences of harvest selection, where small-bodied, early-reproducing individuals are immedia... |  | Biodiversity, Community ecology, Competition, Eco-evolutionary dynamics, Evolutionary ecology, Food webs, Interaction networks, Life history, Population ecology, Theoretical ecology | Simon Blanchet | 2020-04-03 16:14:05 | View | |
12 Oct 2020

Insect herbivory on urban trees: Complementary effects of tree neighbours and predationAlex Stemmelen, Alain Paquette, Marie-Lise Benot, Yasmine Kadiri, Hervé Jactel, Bastien Castagneyrol https://doi.org/10.1101/2020.04.15.042317Tree diversity is associated with reduced herbivory in urban forestRecommended by Ruth Arabelle Hufbauer and Ian Pearse based on reviews by Ian Pearse and Freerk MollemanUrban ecology, the study of ecological systems in our increasingly urbanized world, is crucial to planning and redesigning cities to enhance ecosystem services (Kremer et al. 2016), human health and well-being and further conservation goals (Dallimer et al. 2012). Urban trees are a crucial component of urban streets and parks that provide shade and cooling through evapotranspiration (Fung and Jim 2019), improve air quality (Lai and Kontokosta 2019), help control storm water (Johnson and Handel 2016), and conserve wildlife (Herrmann et al. 2012; de Andrade et al. 2020). References Airola, D. and Greco, S. (2019). Birds and oaks in California’s urban forest. Int. Oaks, 30, 109–116. | Insect herbivory on urban trees: Complementary effects of tree neighbours and predation | Alex Stemmelen, Alain Paquette, Marie-Lise Benot, Yasmine Kadiri, Hervé Jactel, Bastien Castagneyrol | <p>Insect herbivory is an important component of forest ecosystems functioning and can affect tree growth and survival. Tree diversity is known to influence insect herbivory in natural forest, with most studies reporting a decrease in herbivory wi... |  | Biodiversity, Biological control, Community ecology, Ecosystem functioning, Herbivory | Ruth Arabelle Hufbauer | 2020-04-20 13:49:36 | View | |
30 Sep 2020
How citizen science could improve Species Distribution Models and their independent assessmentFlorence Matutini, Jacques Baudry, Guillaume Pain, Morgane Sineau, Josephine Pithon https://doi.org/10.1101/2020.06.02.129536Citizen science contributes to SDM validationRecommended by Francisco Lloret based on reviews by Maria Angeles Perez-Navarro and 1 anonymous reviewerCitizen science is becoming an important piece for the acquisition of scientific knowledge in the fields of natural sciences, and particularly in the inventory and monitoring of biodiversity (McKinley et al. 2017). The information generated with the collaboration of citizens has an evident importance in conservation, by providing information on the state of populations and habitats, helping in mitigation and restoration actions, and very importantly contributing to involve society in conservation (Brown and Williams 2019).
An obvious advantage of these initiatives is the ability to mobilize human resources on a large territorial scale and in the medium term, which would otherwise be difficult to finance. The resulting increasing information then can be processed with advanced computational techniques (Hochachka et al 2012; Kelling et al. 2015), thus improving our interpretation of the distribution of species. Specifically, the ability to obtain information on a large territorial scale can be integrated into studies based on Species Distribution Models SDMs. One of the common problems with SDMs is that they often work from species occurrences that have been opportunistically recorded, either by professionals or amateurs. A great challenge for data obtained from non-professional citizens, however, remains to ensure its standardization and quality (Kosmala et al. 2016). This requires a clear and effective design, solid volunteer training, and a high level of coordination that turns out to be complex (Brown and Williams 2019). Finally, it is essential to perform a quality validation following scientifically recognized standards, since they are often conditioned by errors and biases in obtaining information (Bird et al. 2014). There are two basic approaches to obtain the necessary data for this validation: getting it from an external source (external validation), or allocating a part of the database itself (internal validation or cross-validation) to this function. References [1] Bird TJ et al. (2014) Statistical solutions for error and bias in global citizen science datasets. Biological Conservation 173: 144-154. doi: 10.1016/j.biocon.2013.07.037 | How citizen science could improve Species Distribution Models and their independent assessment | Florence Matutini, Jacques Baudry, Guillaume Pain, Morgane Sineau, Josephine Pithon | <p>Species distribution models (SDM) have been increasingly developed in recent years but their validity is questioned. Their assessment can be improved by the use of independent data but this can be difficult to obtain and prohibitive to collect.... | Biodiversity, Biogeography, Conservation biology, Habitat selection, Spatial ecology, Metacommunities & Metapopulations, Species distributions, Statistical ecology | Francisco Lloret | 2020-06-03 09:36:34 | View | ||
28 Sep 2020
The dynamics of spawning acts by a semelparous fish and its associated energetic costsCédric Tentelier, Colin Bouchard, Anaïs Bernardin, Amandine Tauzin, Jean-Christophe Aymes, Frédéric Lange, Charlotte Recapet, Jacques Rives https://doi.org/10.1101/436295Extreme weight loss: when accelerometer could reveal reproductive investment in a semelparous fish speciesRecommended by Francois-Xavier Dechaume-Moncharmont based on reviews by Aidan Jonathan Mark Hewison, Loïc Teulier and 1 anonymous reviewer based on reviews by Aidan Jonathan Mark Hewison, Loïc Teulier and 1 anonymous reviewer
Continuous observation of animal behaviour could be quite a challenge in the field, and the situation becomes even more complicated with aquatic species mostly active at night. In such cases, biologging techniques are real game changers in ecology, behavioural ecology or eco-physiology. An accelerating number of methodological applications of these tools in natural condition are thus published each year [1]. Biologging is not limited to movement ecology. For instance, fine grain information about energy expenditure can be inferred from body acceleration [2], and accelerometers has already proven useful in monitoring reproductive costs in some fish species [3,4]. The first part of the study by Tentelier et al. [5] is in line with this growing literature. It describes measurements of energy expenditure during reproduction in a fish species, Allis shad (Alosa Alosa), based on tail beat frequency and occurrence of spawning acts. The study has been convincingly conducted, and the results are important for fish biologists. But this is not the whole story: the authors added to this otherwise classical study a very original and insightful analysis which deserves closer interest. References [1] Börger L, Bijleveld AI, Fayet AL, Machovsky‐Capuska GE, Patrick SC, Street GM and Vander Wal E. (2020) Biologging special feature. J. Anim. Ecol. 89, 6–15. 10.1111/1365-2656.13163 | The dynamics of spawning acts by a semelparous fish and its associated energetic costs | Cédric Tentelier, Colin Bouchard, Anaïs Bernardin, Amandine Tauzin, Jean-Christophe Aymes, Frédéric Lange, Charlotte Recapet, Jacques Rives | <p>1. During the reproductive season, animals have to manage both their energetic budget and gamete stock. In particular, for semelparous capital breeders with determinate fecundity and no parental care other than gametic investment, the depletion... | Behaviour & Ethology, Freshwater ecology, Life history | Francois-Xavier Dechaume-Moncharmont | 2020-06-04 15:18:56 | View | ||
21 Dec 2020

Influence of local landscape and time of year on bat-road collision risksCharlotte Roemer, Aurélie Coulon, Thierry Disca, and Yves Bas https://doi.org/10.1101/2020.07.15.204115Assessing bat-vehicle collision risks using acoustic 3D trackingRecommended by Gloriana Chaverri based on reviews by Mark Brigham and ? based on reviews by Mark Brigham and ?
The loss of biodiversity is an issue of great concern, especially if the extinction of species or the loss of a large number of individuals within populations results in a loss of critical ecosystem services. We know that the most important threat to most species is habitat loss and degradation (Keil et al., 2015; Pimm et al., 2014); the latter can be caused by multiple anthropogenic activities, including pollution, introduction of invasive species and fragmentation (Brook et al., 2008; Scanes, 2018). Roads are a major cause of habitat fragmentation, isolating previously connected populations and being a direct source of mortality for animals that attempt to cross them (Spellberg, 1998). References [1] Bartonička T, Andrášik R, Duľa M, Sedoník J, Bíl M (2018) Identification of local factors causing clustering of animal-vehicle collisions. The Journal of Wildlife Management, 82, 940–947. https://doi.org/10.1002/jwmg.21467 | Influence of local landscape and time of year on bat-road collision risks | Charlotte Roemer, Aurélie Coulon, Thierry Disca, and Yves Bas | <p>Roads impact bat populations through habitat loss and collisions. High quality habitats particularly increase bat mortalities on roads, yet many questions remain concerning how local landscape features may influence bat behaviour and lead to hi... |  | Behaviour & Ethology, Biodiversity, Conservation biology, Human impact, Landscape ecology | Gloriana Chaverri | 2020-07-20 10:56:29 | View | |
18 Dec 2020

Once upon a time in the far south: Influence of local drivers and functional traits on plant invasion in the harsh sub-Antarctic islandsManuele Bazzichetto, François Massol, Marta Carboni, Jonathan Lenoir, Jonas Johan Lembrechts, Rémi Joly, David Renault https://doi.org/10.1101/2020.07.19.210880A meaningful application of species distribution models and functional traits to understand invasion dynamicsRecommended by Joaquín Hortal based on reviews by Paula Matos and Peter ConveyPolar and subpolar regions are fragile environments, where the introduction of alien species may completely change ecosystem dynamics if the alien species become keystone species (e.g. Croll, 2005). The increasing number of human visits, together with climate change, are favouring the introduction and settling of new invaders to these regions, particularly in Antarctica (Hughes et al. 2015). Within this context, the joint use of Species Distribution Models (SDM) –to assess the areas potentially suitable for the aliens– with other measures of the potential to become successful invaders can inform on the need for devoting specific efforts to eradicate these new species before they become naturalized (e.g. Pertierra et al. 2016). References Austin, M. P., Nicholls, A. O., and Margules, C. R. (1990). Measurement of the realized qualitative niche: environmental niches of five Eucalyptus species. Ecological Monographs, 60(2), 161-177. doi: https://doi.org/10.2307/1943043 | Once upon a time in the far south: Influence of local drivers and functional traits on plant invasion in the harsh sub-Antarctic islands | Manuele Bazzichetto, François Massol, Marta Carboni, Jonathan Lenoir, Jonas Johan Lembrechts, Rémi Joly, David Renault | <p>Aim Here, we aim to: (i) investigate the local effect of environmental and human-related factors on alien plant invasion in sub-Antarctic islands; (ii) explore the relationship between alien species features and their dependence on anthropogeni... |  | Biogeography, Biological invasions, Spatial ecology, Metacommunities & Metapopulations, Species distributions | Joaquín Hortal | 2020-07-21 21:13:08 | View |
MANAGING BOARD
Julia Astegiano
Tim Coulson
Anna Eklof
Dominique Gravel
François Massol
Ben Phillips
Cyrille Violle










