Latest recommendations

| Id | Title | Authors | Abstract | Picture | Thematic fields | Recommender | Reviewers▲ | Submission date | |
|---|---|---|---|---|---|---|---|---|---|
22 May 2019

Sex makes them sleepy: host reproductive status induces diapause in a parasitoid population experiencing harsh wintersTougeron K., Brodeur J., van Baaren J., Renault D. and Le Lann C. https://doi.org/10.1101/371385The response of interacting species to biotic seasonal cuesRecommended by Adele Mennerat and Enric Frago based on reviews by Anne Duplouy and 1 anonymous reviewerIn temperate regions, food abundance and quality vary greatly throughout the year, and the ability of organisms to synchronise their phenology to these changes is a key determinant of their reproductive success. Successful synchronisation requires that cues are perceived prior to change, leaving time for physiological adjustments. References [1] Tauber, M. J., Tauber, C. A., and Masaki, S. (1986). Seasonal Adaptations of Insects. Oxford, New York: Oxford University Press. | Sex makes them sleepy: host reproductive status induces diapause in a parasitoid population experiencing harsh winters | Tougeron K., Brodeur J., van Baaren J., Renault D. and Le Lann C. | <p>When organisms coevolve, any change in one species can induce phenotypic changes in traits and ecology of the other species. The role such interactions play in ecosystems is central, but their mechanistic bases remain underexplored. Upper troph... |  | Coexistence, Evolutionary ecology, Experimental ecology, Host-parasite interactions, Physiology | Adele Mennerat | 2018-07-18 18:51:03 | View | |
08 Jan 2020

Studies of NH4+ and NO3- uptake ability of subalpine plants and resource-use strategy identified by their functional traitsLegay Nicolas, Grassein Fabrice, Arnoldi Cindy, Segura Raphaël, Laîné Philippe, Lavorel Sandra, Clément Jean-Christophe https://doi.org/10.1101/372235Nitrate or not nitrate. That is the questionRecommended by Sébastien Barot based on reviews by Vincent Maire and 1 anonymous reviewerThe article by Legay et al. [1] addresses two main issues: the links between belowground and aboveground plant traits and the links between plant strategies (as defined by these traits) and the capacity to absorb nitrate and ammonium. I recommend this work because these are important and current issues. The literature on plant traits is extremely rich and the existence of a leaf economic spectrum linked to a gradient between conservative and acquisitive plants is now extremely well established [2-3]. Many teams are now working on belowground traits and possible links with the aboveground gradients [4-5]. It seems indeed that there is a root economic spectrum but this spectrum is apparently less pronounced than the leaf economic spectrum. The existence of links between the two spectrums are still controversial and are likely not universal as suggested by discrepant results and after all a plant could have a conservative strategy aboveground and an acquisitive strategy belowground (or vice-versa) because, indeed, constraints are different belowground and aboveground (for example because in given ecosystem/vegetation type light may be abundant but not water or mineral nutrients). The various results obtained also suggest that we do not full understand the diversity of belowground strategies, what is at stake with these strategies, and the links with root characteristics. References [1] Legay, N., Grassein, F., Arnoldi, C., Segura, R., Laîné, P., Lavorel, S. and Clément, J.-C. (2020). Studies of NH4+ and NO3- uptake ability of subalpine plants and resource-use strategy identified by their functional traits. bioRxiv, 372235, ver. 4 peer-reviewed and recommended by PCI Ecology. doi: 10.1101/372235 | Studies of NH4+ and NO3- uptake ability of subalpine plants and resource-use strategy identified by their functional traits | Legay Nicolas, Grassein Fabrice, Arnoldi Cindy, Segura Raphaël, Laîné Philippe, Lavorel Sandra, Clément Jean-Christophe | <p>The leaf economics spectrum (LES) is based on a suite of leaf traits related to plant functioning and ranges from resource-conservative to resource-acquisitive strategies. However, the relationships with root traits, and the associated belowgro... |  | Community ecology, Physiology, Terrestrial ecology | Sébastien Barot | 2018-07-19 14:22:28 | View | |
25 Oct 2021

The taxonomic and functional biogeographies of phytoplankton and zooplankton communities across boreal lakesNicolas F St-Gelais, Richard J Vogt, Paul A del Giorgio, Beatrix E Beisner https://doi.org/10.1101/373332The difficult interpretation of species co-distributionRecommended by Dominique Gravel based on reviews by Anthony Maire and Emilie MackeEcology is the study of the distribution of organisms in space and time and their interactions. As such, there is a tradition of studies relating abiotic environmental conditions to species distribution, while another one is concerned by the effects of consumers on the abundance of their resources. Interestingly, joining the dots appears more difficult than it would suggest: eluding the effect of species interactions on distribution remains one of the greatest challenges to elucidate nowadays (Kissling et al. 2012). Theory suggests that yes, species interactions such as predation and competition should influence range limits (Godsoe et al. 2017), but the common intuition among many biogeographers remains that over large areas such as regions and continents, environmental drivers like temperature and precipitation overwhelm their local effects. Answering this question is of primary importance in the context where species are moving around with climate warming. Inconsistencies in food web structure may arise with asynchronized movements of consumers and their resources, leading to a major disruption in regulation and potentially ecosystem functioning. Solving this problem, however, remains very challenging because we have to rely on observational data since experiments are hard to perform at the biogeographical scale. The study of St-Gelais is an interesting step forward to solve this problem. Their main objective was to assess the strength of the association between phytoplankton and zooplankton communities at a large spatial scale, looking at the spatial covariation of both taxonomic and functional composition. To do so, they undertook a massive survey of more than 100 lakes across three regions of the boreal region of Québec. Species and functional composition were recorded, along with a set of abiotic variables. Classic community ecology at this point. The difficulty they faced was to disentangle the multiple causal relationships involved in the distribution of both trophic levels. Teasing apart bottom-up and top-down forces driving the assembly of plankton communities using observational data is not an easy task. On the one hand, both trophic levels could respond to variations in temperature, nutrient availability and dissolved organic carbon. The interpretation is fairly straightforward if the two levels respond to different factors, but the situation is much more complicated when they do respond similarly. There are potentially three possible underlying scenarios. First, the phyto and zooplankton communities may share the same environmental requirements, thereby generating a joint distribution over gradients such as temperature and nutrient availability. Second, the abiotic environment could drive the distribution of the phytoplankton community, which would then propagate up and influence the distribution of the zooplankton community. Alternatively, the abiotic environment could constrain the distribution of the zooplankton, which could then affect the one of phytoplankton. In addition to all of these factors, St-Gelais et al also consider that dispersal may limit the distribution, well aware of previous studies documenting stronger dispersal limitations for zooplankton communities. Unfortunately, there is not a single statistical approach that could be taken from the shelf and used to elucidate drivers of co-distribution. Joint species distribution was once envisioned as a major step forward in this direction (Warton et al. 2015), but there are several limits preventing the direct interpretation that co-occurrence is linked to interactions (Blanchet et al. 2020). Rather, St-Gelais used a variety of multivariate statistics to reveal the structure in their observational data. First, using a Procrustes analysis (a method testing if the spatial variation of one community is correlated to the structure of another community), they found a significant correlation between phytoplankton and zooplankton communities, indicating a taxonomic coupling between the groups. Interestingly, this observation was maintained for functional composition only when interaction-related traits were considered. At this point, these results strongly suggest that interactions are involved in the correlation, but it's hard to decipher between bottom-up and top-down perspectives. A complementary analysis performed with a constrained ordination, per trophic level, provided complementary pieces of information. First observation was that only functional variation was found to be related to the different environmental variables, not taxonomic variation. Despite that trophic levels responded to water quality variables, spatial autocorrelation was more important for zooplankton communities and the two layers appear to respond to different variables. It is impossible with those results to formulate a strong conclusion about whether grazing influence the co-distribution of phytoplankton and zooplankton communities. That's the mere nature of observational data. While there is a strong spatial association between them, there are also diverging responses to the different environmental variables considered. But the contrast between taxonomic and functional composition is nonetheless informative and it seems that beyond the idiosyncrasies of species composition, trait distribution may be more informative and general. Perhaps the most original contribution of this study is the hierarchical approach to analyze the data, combined with the simultaneous analysis of taxonomic and functional distributions. Having access to a vast catalog of multivariate statistical techniques, a careful selection of analyses helps revealing key features in the data, rejecting some hypotheses and accepting others. Hopefully, we will see more and more of such multi-trophic approaches to distribution because it is now clear that the factors driving distribution are much more complicated than anticipated in more traditional analyses of community data. Biodiversity is more than a species list, it is also all of the interactions between them, influencing their distribution and abundance (Jordano 2016). References Blanchet FG, Cazelles K, Gravel D (2020) Co-occurrence is not evidence of ecological interactions. Ecology Letters, 23, 1050–1063. https://doi.org/10.1111/ele.13525 Godsoe W, Jankowski J, Holt RD, Gravel D (2017) Integrating Biogeography with Contemporary Niche Theory. Trends in Ecology & Evolution, 32, 488–499. https://doi.org/10.1016/j.tree.2017.03.008 Jordano P (2016) Chasing Ecological Interactions. PLOS Biology, 14, e1002559. https://doi.org/10.1371/journal.pbio.1002559 Kissling WD, Dormann CF, Groeneveld J, Hickler T, Kühn I, McInerny GJ, Montoya JM, Römermann C, Schiffers K, Schurr FM, Singer A, Svenning J-C, Zimmermann NE, O’Hara RB (2012) Towards novel approaches to modelling biotic interactions in multispecies assemblages at large spatial extents. Journal of Biogeography, 39, 2163–2178. https://doi.org/10.1111/j.1365-2699.2011.02663.x St-Gelais NF, Vogt RJ, Giorgio PA del, Beisner BE (2021) The taxonomic and functional biogeographies of phytoplankton and zooplankton communities across boreal lakes. bioRxiv, 373332, ver. 4 peer-reviewed and recommended by Peer community in Ecology. https://doi.org/10.1101/373332 Warton DI, Blanchet FG, O’Hara RB, Ovaskainen O, Taskinen S, Walker SC, Hui FKC (2015) So Many Variables: Joint Modeling in Community Ecology. Trends in Ecology & Evolution, 30, 766–779. https://doi.org/10.1016/j.tree.2015.09.007 Wisz MS, Pottier J, Kissling WD, Pellissier L, Lenoir J, Damgaard CF, Dormann CF, Forchhammer MC, Grytnes J-A, Guisan A, Heikkinen RK, Høye TT, Kühn I, Luoto M, Maiorano L, Nilsson M-C, Normand S, Öckinger E, Schmidt NM, Termansen M, Timmermann A, Wardle DA, Aastrup P, Svenning J-C (2013) The role of biotic interactions in shaping distributions and realised assemblages of species: implications for species distribution modelling. Biological Reviews, 88, 15–30. https://doi.org/10.1111/j.1469-185X.2012.00235.x | The taxonomic and functional biogeographies of phytoplankton and zooplankton communities across boreal lakes | Nicolas F St-Gelais, Richard J Vogt, Paul A del Giorgio, Beatrix E Beisner | <p>Strong trophic interactions link primary producers (phytoplankton) and consumers (zooplankton) in lakes. However, the influence of such interactions on the biogeographical distribution of the taxa and functional traits of planktonic organ... |  | Biogeography, Community ecology, Species distributions | Dominique Gravel | 2018-07-24 15:01:51 | View | |
10 Jan 2019

Inferring macro-ecological patterns from local species' occurrencesAnna Tovo, Marco Formentin, Samir Suweis, Samuele Stivanello, Sandro Azaele, Amos Maritan https://doi.org/10.1101/387456Upscaling the neighborhood: how to get species diversity, abundance and range distributions from local presence/absence dataRecommended by Matthieu Barbier based on reviews by Kevin Cazelles and 1 anonymous reviewer based on reviews by Kevin Cazelles and 1 anonymous reviewer
How do you estimate the biodiversity of a whole community, or the distribution of abundances and ranges of its species, from presence/absence data in scattered samples? ADDITIONAL COMMENTS 1) To explain the novelty of the authors' contribution, it is useful to look at competing techniques. 2) The main condition for all such approaches to work is well-mixedness: each sample should be sufficiently like a lot drawn from the same skewed lottery. As long as that condition applies, finding the best approach is a theoretical matter of probabilities and combinatorics that may, in time, be given a definite answer. 3) One may ask: why the Negative Binomial as a Species Abundance Distribution? References [1] Fisher, R. A., Corbet, A. S., & Williams, C. B. (1943). The relation between the number of species and the number of individuals in a random sample of an animal population. The Journal of Animal Ecology, 42-58. doi: 10.2307/1411 | Inferring macro-ecological patterns from local species' occurrences | Anna Tovo, Marco Formentin, Samir Suweis, Samuele Stivanello, Sandro Azaele, Amos Maritan | <p>Biodiversity provides support for life, vital provisions, regulating services and has positive cultural impacts. It is therefore important to have accurate methods to measure biodiversity, in order to safeguard it when we discover it to be thre... |  | Macroecology, Species distributions, Statistical ecology, Theoretical ecology | Matthieu Barbier | 2018-08-09 16:44:09 | View | |
31 Jan 2019
Do the more flexible individuals rely more on causal cognition? Observation versus intervention in causal inference in great-tailed gracklesAaron Blaisdell, Zoe Johnson-Ulrich, Luisa Bergeron, Carolyn Rowney, Benjamin Seitz, Kelsey McCune, Corina Logan http://corinalogan.com/Preregistrations/g_causal.htmlFrom cognition to range dynamics: advancing our understanding of macroecological patternsRecommended by Emanuel A. Fronhofer based on reviews by 2 anonymous reviewersUnderstanding the distribution of species on earth is one of the fundamental challenges in ecology and evolution. For a long time, this challenge has mainly been addressed from a correlative point of view with a focus on abiotic factors determining a species abiotic niche (classical bioenvelope models; [1]). It is only recently that researchers have realized that behaviour and especially plasticity in behaviour may play a central role in determining species ranges and their dynamics [e.g., 2-5]. Blaisdell et al. propose to take this even one step further and to analyse how behavioural flexibility and possibly associated causal cognition impacts range dynamics. References | Do the more flexible individuals rely more on causal cognition? Observation versus intervention in causal inference in great-tailed grackles | Aaron Blaisdell, Zoe Johnson-Ulrich, Luisa Bergeron, Carolyn Rowney, Benjamin Seitz, Kelsey McCune, Corina Logan | This PREREGISTRATION has undergone one round of peer reviews. We have now revised the preregistration and addressed reviewer comments. The DOI was issued by OSF and refers to the whole GitHub repository, which contains multiple files. The specific... | Behaviour & Ethology, Preregistrations, Zoology | Emanuel A. Fronhofer | 2018-08-20 11:09:48 | View | ||
04 Sep 2019
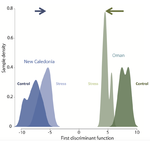
Gene expression plasticity and frontloading promote thermotolerance in Pocillopora coralsK. Brener-Raffalli, J. Vidal-Dupiol, M. Adjeroud, O. Rey, P. Romans, F. Bonhomme, M. Pratlong, A. Haguenauer, R. Pillot, L. Feuillassier, M. Claereboudt, H. Magalon, P. Gélin, P. Pontarotti, D. Aurelle, G. Mitta, E. Toulza https://doi.org/10.1101/398602Transcriptomics of thermal stress response in coralsRecommended by Staffan Jacob based on reviews by Mar SobralClimate change presents a challenge to many life forms and the resulting loss of biodiversity will critically depend on the ability of organisms to timely respond to a changing environment. Shifts in ecological parameters have repeatedly been attributed to global warming, with the effectiveness of these responses varying among species [1, 2]. Organisms do not only have to face a global increase in mean temperatures, but a complex interplay with another crucial but largely understudied aspect of climate change: thermal fluctuations. Understanding the mechanisms underlying adaptation to thermal fluctuations is thus a timely and critical challenge. References [1] Parmesan, C., & Yohe, G. (2003). A globally coherent fingerprint of climate change impacts across natural systems. Nature, 421(6918), 37–42. doi: 10.1038/nature01286 | Gene expression plasticity and frontloading promote thermotolerance in Pocillopora corals | K. Brener-Raffalli, J. Vidal-Dupiol, M. Adjeroud, O. Rey, P. Romans, F. Bonhomme, M. Pratlong, A. Haguenauer, R. Pillot, L. Feuillassier, M. Claereboudt, H. Magalon, P. Gélin, P. Pontarotti, D. Aurelle, G. Mitta, E. Toulza | <p>Ecosystems worldwide are suffering from climate change. Coral reef ecosystems are globally threatened by increasing sea surface temperatures. However, gene expression plasticity provides the potential for organisms to respond rapidly and effect... |  | Climate change, Evolutionary ecology, Marine ecology, Molecular ecology, Phenotypic plasticity, Symbiosis | Staffan Jacob | 2018-08-29 10:46:55 | View | |
14 May 2019
Field assessment of precocious maturation in salmon parr using ultrasound imagingMarie Nevoux, Frédéric Marchand, Guillaume Forget, Dominique Huteau, Julien Tremblay, Jean-Pierre Destouches https://doi.org/10.1101/425561OB-GYN for salmon parrsRecommended by Jean-Olivier Irisson based on reviews by Hervé CAPRA and 1 anonymous reviewer based on reviews by Hervé CAPRA and 1 anonymous reviewer
Population dynamics and stock assessment models are only as good as the data used to parameterise them. For Atlantic salmon (Salmo salar) populations, a critical parameter may be frequency of precocious maturation. Indeed, the young males (parrs) that mature early, before leaving the river to reach the ocean, can contribute to reproduction but have much lower survival rates afterwards. The authors cite evidence of the potentially major consequences of this alternate reproductive strategy. So, to be parameterised correctly, it needs to be assessed correctly. Cue the ultrasound machine. Through a thorough analysis of data collected on 850 individuals [1], over three years, the authors clearly show that the non-invasive examination of the internal cavity of young fishes to look for gonads, using a portable ultrasound machine, provides reliable and replicable evidence of precocious maturation. They turned into OB-GYN for salmons (albeit for male salmons!) and it worked. While using ultrasounds to detect fish gonads is not a new idea (early attempts for salmonids date back to the 80s [2]), the value here is in the comparison with the classic visual inspection technique (which turns out to be less reliable) and the fact that ultrasounds can now easily be carried out in the field. Beyond the potentially important consequences of this new technique for the correct assessment of salmon population dynamics, the authors also make the case for the acquisition of more reliable individual-level data in ecological studies, which I applaud. References. [1] Nevoux M, Marchand F, Forget G, Huteau D, Tremblay J, and Destouches J-P. (2019). Field assessment of precocious maturation in salmon parr using ultrasound imaging. bioRxiv 425561, ver. 3 peer-reviewed and recommended by PCI Ecology. doi: 10.1101/425561 | Field assessment of precocious maturation in salmon parr using ultrasound imaging | Marie Nevoux, Frédéric Marchand, Guillaume Forget, Dominique Huteau, Julien Tremblay, Jean-Pierre Destouches | <p>Salmonids are characterized by a large diversity of life histories, but their study is often limited by the imperfect observation of the true state of an individual in the wild. Challenged by the need to reduce uncertainty of empirical data, re... | Conservation biology, Demography, Experimental ecology, Freshwater ecology, Life history, Phenotypic plasticity, Population ecology | Jean-Olivier Irisson | 2018-09-25 17:24:59 | View | ||
26 Mar 2019
Is behavioral flexibility linked with exploration, but not boldness, persistence, or motor diversity?Kelsey McCune, Carolyn Rowney, Luisa Bergeron, Corina Logan http://corinalogan.com/Preregistrations/g_exploration.htmlProbing behaviors correlated with behavioral flexibilityRecommended by Jeremy Van Cleve based on reviews by 2 anonymous reviewersBehavioral plasticity, which is a subset of phenotypic plasticity, is an important component of foraging, defense against predators, mating, and many other behaviors. More specifically, behavioral flexibility, in this study, captures how quickly individuals adapt to new circumstances. In cases where individuals disperse to new environments, which often occurs in range expansions, behavioral flexibility is likely crucial to the chance that individuals can establish in these environments. Thus, it is important to understand how best to measure behavioral flexibility and how measures of such flexibility might vary across individuals and behavioral contexts and with other measures of learning and problem solving. | Is behavioral flexibility linked with exploration, but not boldness, persistence, or motor diversity? | Kelsey McCune, Carolyn Rowney, Luisa Bergeron, Corina Logan | This is a PREREGISTRATION. The DOI was issued by OSF and refers to the whole GitHub repository, which contains multiple files. The specific file we are submitting is g_exploration.Rmd, which is easily accessible at GitHub at https://github.com/cor... | Behaviour & Ethology, Preregistrations, Zoology | Jeremy Van Cleve | 2018-09-27 03:35:12 | View | ||
01 Apr 2019
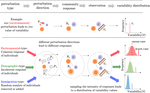
The inherent multidimensionality of temporal variability: How common and rare species shape stability patternsJean-François Arnoldi, Michel Loreau, Bart Haegeman https://doi.org/10.1101/431296Diversity-Stability and the Structure of PerturbationsRecommended by Kevin Cazelles and Kevin Shear McCann based on reviews by Frederic Barraquand and 1 anonymous reviewer and Kevin Shear McCann based on reviews by Frederic Barraquand and 1 anonymous reviewer
In his 1972 paper “Will a Large Complex System Be Stable?” [1], May challenges the idea that large communities are more stable than small ones. This was the beginning of a fundamental debate that still structures an entire research area in ecology: the diversity-stability debate [2]. The most salient strength of May’s work was to use a mathematical argument to refute an idea based on the observations that simple communities are less stable than large ones. Using the formalism of dynamical systems and a major results on the distribution of the eigen values for random matrices, May demonstrated that the addition of random interactions destabilizes ecological communities and thus, rich communities with a higher number of interactions should be less stable. But May also noted that his mathematical argument holds true only if ecological interactions are randomly distributed and thus concluded that this must not be true! This is how the contradiction between mathematics and empirical observations led to new developments in the study of ecological networks. References [1] May, Robert M (1972). Will a Large Complex System Be Stable? Nature 238, 413–414. doi: 10.1038/238413a0 | The inherent multidimensionality of temporal variability: How common and rare species shape stability patterns | Jean-François Arnoldi, Michel Loreau, Bart Haegeman | <p>Empirical knowledge of ecosystem stability and diversity-stability relationships is mostly based on the analysis of temporal variability of population and ecosystem properties. Variability, however, often depends on external factors that act as... |  | Biodiversity, Coexistence, Community ecology, Competition, Interaction networks, Theoretical ecology | Kevin Cazelles | 2018-10-02 14:01:03 | View | |
05 Apr 2019

Using a large-scale biodiversity monitoring dataset to test the effectiveness of protected areas at conserving North-American breeding birdsVictor Cazalis, Soumaya Belghali, Ana S.L. Rodrigues https://doi.org/10.1101/433037Protected Areas effects on biodiversity: a test using bird data that hopefully will give ideas for much more studies to comeRecommended by Paul Caplat based on reviews by Willson Gaul and 1 anonymous reviewerIn the face of worldwide declines in biodiversity, evaluating the effectiveness of conservation practices is an absolute necessity. Protected Areas (PA) are a key tool for conservation, and the question “Are PA effective” has been on many a research agenda, as the introduction to this preprint will no doubt convince you. A challenge we face is that, until now, few studies have been explicitly designed to evaluate PA, and despite the rise of meta-analyses on the topic, our capacity to quantify their effect on biodiversity remains limited. References [1] Cazalis, V., Belghali, S., & Rodrigues, A. S. (2019). Using a large-scale biodiversity monitoring dataset to test the effectiveness of protected areas at conserving North-American breeding birds. bioRxiv, 433037, ver. 4 peer-reviewed and recommended by PCI Ecology. doi: 10.1101/433037 | Using a large-scale biodiversity monitoring dataset to test the effectiveness of protected areas at conserving North-American breeding birds | Victor Cazalis, Soumaya Belghali, Ana S.L. Rodrigues | <p>Protected areas currently cover about 15% of the global land area, and constitute one of the main tools in biodiversity conservation. Quantifying their effectiveness at protecting species from local decline or extinction involves comparing prot... |  | Biodiversity, Conservation biology, Human impact, Landscape ecology, Macroecology | Paul Caplat | 2018-10-04 08:43:34 | View |
MANAGING BOARD
Julia Astegiano
Tim Coulson
Anna Eklof
Dominique Gravel
François Massol
Ben Phillips
Cyrille Violle










