Latest recommendations

| Id | Title | Authors | Abstract | Picture | Thematic fields | Recommender▼ | Reviewers | Submission date | |
|---|---|---|---|---|---|---|---|---|---|
07 Jun 2023
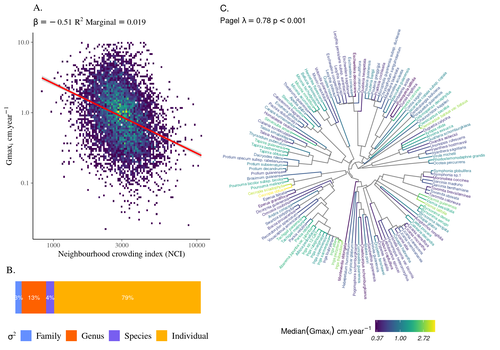
High intraspecific growth variability despite strong evolutionary heritage in a neotropical forestSylvain Schmitt, Bruno Hérault, Géraldine Derroire https://doi.org/10.1101/2022.07.27.501745Environmental and functional determinants of tree performance in a neotropical forest: the imprint of evolutionary legacy on growth strategiesRecommended by François Munoz based on reviews by David Murray-Stoker, Camille Girard and Jelena Pantel based on reviews by David Murray-Stoker, Camille Girard and Jelena Pantel
The hyperdiverse tropical forests have long fascinated ecologists because the fact that so many species persist at a low density at a local scale remains hard to explain. Both niche-based and neutral hypotheses have been tested, primarily based on analyzing the taxonomic composition of tropical forest plots (Janzen 1970; Hubbell 2001). Studies of the functional and phylogenetic structure of tropical tree communities have further aimed to better assess the importance of niche-based processes. For instance, Baraloto et al. (2012) found that co-occurring species were functionally and phylogenetically more similar in a neotropical forest, suggesting a role of environmental filtering. Likewise, Schmitt et al. (2021) found the influence of environmental filtering on the functional composition of an Indian rainforest. Yet these studies evidenced non-random trait-environment association based on the composition of assemblages only (in terms of occurrences and abundances). A major challenge remains to further address whether and how tree performance varies among species and individuals in tropical forests. Functional traits are related to components of individual fitness (Violle et al. 2007). Recently, more and more emphasis has been put on examining the relationship between functional trait values and demographic parameters (Salguero-Gómez et al. 2018), in order to better understand how functional trait values determine species population dynamics and abundances in assemblages. Fortunel et al. (2018) found an influence of functional traits on species growth variation related to topography, and less clearly to neighborhood density (crowding). Poorter et al. (2018) observed 44% of trait variation within species in a neotropical forest. Although individual trait values would be expected to be better predictors of performance than average values measured at the species level, Poorter et al still found a poor relationship. Schmitt et al. (2023) examined how abiotic conditions and biotic interactions (considering neighborhood density) influenced the variation of individual potential tree growth, in a tropical forest plot located in French Guiana. They also considered the link between species-averaged values of growth potential and functional traits. Schmitt et al. (2023) found substantial variation in growth potential within species, that functional traits explained 40% of the variation of species-averaged growth and, noticeably, that the taxonomic structure (used as random effect in their model) explained a third of the variation in individual growth. Although functional traits of roots, wood and leaves could predict a significant part of species growth potential, much variability of tree growth occurred within species. Intraspecific trait variation can thus be huge in response to changing abiotic and biotic contexts across individuals. The information on phylogenetic relationships can still provide a proxy of the integrated phenotypic variation that is under selection across the phylogeny, and determine a variation in growth strategies among individuals. The similarity of the phylogenetic structure suggests a joint selection of these growth strategies and related functional traits during events of convergent evolution. Baraloto et al. (2012) already noted that phylogenetic distance can be a proxy of niche overlap in tropical tree communities. Here, Schmitt et al. further demonstrate that evolutionary heritage is significantly related to individual growth variation, and plead for better acknowledging this role in future studies. While the role of fitness differences in tropical tree community dynamics remained to be assessed, the present study provides new evidence that individual growth does vary depending on evolutionary relationships, which can reflect the roles of selection and adaptation on growth strategies. Therefore, investigating both the influence of functional traits and phylogenetic relationships on individual performance remains a promising avenue of research, for functional and community ecology in general. REFERENCES Baraloto, Christopher, Olivier J. Hardy, C. E. Timothy Paine, Kyle G. Dexter, Corinne Cruaud, Luke T. Dunning, Mailyn-Adriana Gonzalez, et al. 2012. « Using functional traits and phylogenetic trees to examine the assembly of tropical tree communities ». Journal of Ecology, 100: 690‑701. | High intraspecific growth variability despite strong evolutionary heritage in a neotropical forest | Sylvain Schmitt, Bruno Hérault, Géraldine Derroire | <p style="text-align: justify;">Individual tree growth is a key determinant of species performance and a driver of forest dynamics and composition. Previous studies on tree growth unravelled the variation in species growth as a function of demogra... |  | Community ecology, Demography, Population ecology | François Munoz | Jelena Pantel, David Murray-Stoker | 2022-08-01 14:29:04 | View |
13 May 2024
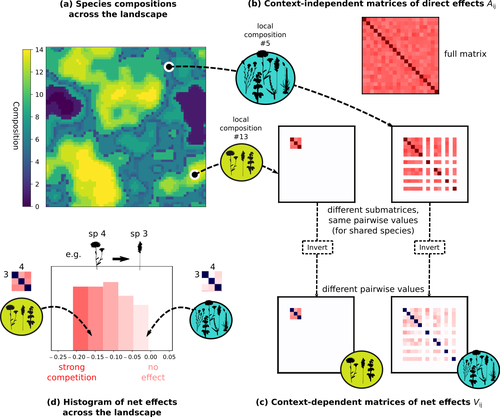
Getting More by Asking for Less: Linking Species Interactions to Species Co-Distributions in MetacommunitiesMatthieu Barbier, Guy Bunin, Mathew A. Leibold https://doi.org/10.1101/2023.06.04.543606Beyond pairwise species interactions: coarser inference of their joined effects is more relevantRecommended by François Munoz based on reviews by Frederik De Laender, Hao Ran Lai and Malyon Bimler based on reviews by Frederik De Laender, Hao Ran Lai and Malyon Bimler
Barbier et al. (2024) investigated the dynamics of species abundances depending on their ecological niche (abiotic component) and on (numerous) competitive interactions. In line with previous evidence and expectations (Barbier et al. 2018), the authors show that it is possible to robustly infer the mean and variance of interaction coefficients from species co-distributions, while it is not possible to infer the individual coefficient values. The authors devised a simulation framework representing multispecies dynamics in an heterogeneous environmental context (2D grid landscape). They used a Lotka-Volterra framework involving pairwise interaction coefficients and species-specific carrying capacities. These capacities depend on how well the species niche matches the local environmental conditions, through a Gaussian function of the distance of the species niche centers to the local environmental values. They considered two contrasted scenarios denoted as « Environmental tracking » and « Dispersal limited ». In the latter case, species are initially seeded over the environmental grid and cannot disperse to other cells, while in the former case they can disperse and possibly be more performant in other cells. The direct effects of species on one another are encoded in an interaction matrix A, and the authors further considered net interactions depending on the inverse of the matrix of direct interactions (Zelnik et al., 2024). The net effects are context-dependent, i.e., it involves the environment-dependent biotic capacities, even through the interaction terms can be defined between species as independent from local environment. The results presented here underline that the outcome of many individual competitive interactions can only be understood in terms of macroscopic properties. In essence, the results here echoe the mean field theories that investigate the dynamics of average ecological properties instead of the microscopic components (e.g., McKane et al. 2000). In a philosophical perspective, community ecology has long struggled with analyzing and inferring local determinants of species coexistence from species co-occurrence patterns, so that it was claimed that no universal laws can be derived in the discipline (Lawton 1999). Using different and complementary methods and perspectives, recent research has also shown that species assembly parameter values cannot be unambiguously inferred from species co-occurrences only, even in simple designs where an equilibrium can be reached (Poggiato et al. 2021). Although the roles of high-order competitive interactions and intransivity can lead to species coexistence, the simple view of a single loop of competitive interactions is easily challenged when further interactions and complexity is added (Gallien et al. 2024). But should we put so much emphasis on inferring individual interaction coefficients? In a quest to understand the emerging properties of elementary processes, ecological theory could go forward with a more macroscopic analysis and understanding of species coexistence in many communities. The authors referred several times to an interesting paper from Schaffer (1981), entitled « Ecological abstraction: the consequences of reduced dimensionality in ecological models ». It proposes that estimating individual species competition coefficients is not possible, but that competition can be assessed at the coarser level of organisation, i.e., between ecological guilds. This idea implies that the dimensionality of the competition equations should be greatly reduced to become tractable in practice. Taking together this claim with the results of the present Barbier et al. (2024) paper, it becomes clearer that the nature of competitive interactions can be addressed through « abstracted » quantities, as those of guilds or the moments of the individual competition coefficients (here the average and the standard deviation). Therefore the scope of Barbier et al. (2024) framework goes beyond statistical issues in parameter inference, but question the way we must think and represent the numerous competitive interactions in a simplified and robust way. References Barbier, Matthieu, Jean-François Arnoldi, Guy Bunin, et Michel Loreau. 2018. « Generic assembly patterns in complex ecological communities ». Proceedings of the National Academy of Sciences 115 (9): 2156‑61. https://doi.org/10.1073/pnas.1710352115 | Getting More by Asking for Less: Linking Species Interactions to Species Co-Distributions in Metacommunities | Matthieu Barbier, Guy Bunin, Mathew A. Leibold | <p>AbstractOne of the more difficult challenges in community ecology is inferring species interactions on the basis of patterns in the spatial distribution of organisms. At its core, the problem is that distributional patterns reflect the ‘realize... |  | Biogeography, Community ecology, Competition, Spatial ecology, Metacommunities & Metapopulations, Species distributions, Statistical ecology, Theoretical ecology | François Munoz | 2023-10-21 14:14:16 | View | |
21 Nov 2023
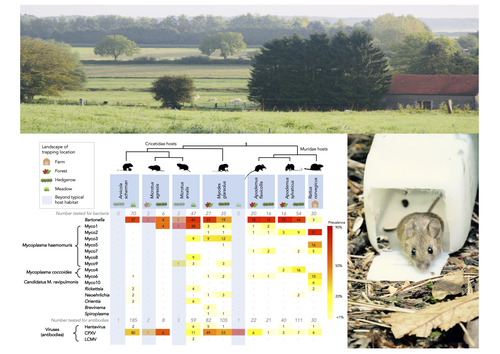
Pathogen community composition and co-infection patterns in a wild community of rodentsJessica Lee Abbate, Maxime Galan, Maria Razzauti, Tarja Sironen, Liina Voutilainen, Heikki Henttonen, Patrick Gasqui, Jean-François Cosson, Nathalie Charbonnel https://doi.org/10.1101/2020.02.09.940494Reservoirs of pestilence: what pathogen and rodent community analyses can tell us about transmission riskRecommended by Francois Massol based on reviews by Adrian Diaz, Romain Pigeault and 1 anonymous reviewer based on reviews by Adrian Diaz, Romain Pigeault and 1 anonymous reviewer
Rodents are well known as one of the main animal groups responsible for human-transmitted pathogens. As such, it seems logical to try and survey what kinds of pathogenic microbes might be harboured by wild rodents, in order to establish some baseline surveillance and prevent future zoonotic outbreaks (Bernstein et al., 2022). This is exactly what Abbate et al. (2023) endeavoured and their findings are intimidating. Based on quite a large sampling effort, they collected more than 700 rodents of seven species around two villages in northeastern France. They looked for molecular markers indicative of viral and bacterial infections and proceeded to analyze their pathogen communities using multivariate techniques. Variation in the prevalence of the different pathogens was found among host species, with e.g. signs of CPXV more prevalent in Cricetidae while some Mycoplasma strains were more prevalent in Muridae. Co-circulation of pathogens was found in all species, with some evidencing signs of up to 12 different pathogen taxa. The diversity of co-circulating pathogens was markedly different between host species and higher in adult hosts, but not affected by sex. The dataset also evinced some slight differences between habitats, with meadows harbouring a little more diversity of rodent pathogens than forests. Less intuitively, some pathogen associations seemed quite repeatable, such as the positive association of Bartonella spp. with CPXV in the montane water vole. The study allowed the authors to test several associations already described in the literature, including associations between different hemotropic Mycoplasma species. I strongly invite colleagues interested in zoonoses, emerging pandemics and more generally One Health to read the paper of Abbate et al. (2023) and try to replicate them across the world. To prevent the next sanitary crises, monitoring rodents, and more generally vertebrates, population demographics is a necessary and enlightening step (Johnson et al., 2020), but insufficient. Following the lead of colleagues working on rodent ectoparasites (Krasnov et al., 2014), we need more surveys like the one described by Abbate et al. (2023) to understand the importance of the dilution effect in the prevalence and transmission of microbial pathogens (Andreazzi et al., 2023) and the formation of epidemics. We also need other similar studies to assess the potential of different rodent species to carry pathogens more or less capable of infecting other mammalian species (Morand et al., 2015), in other places in the world. References Abbate, J. L., Galan, M., Razzauti, M., Sironen, T., Voutilainen, L., Henttonen, H., Gasqui, P., Cosson, J.-F. & Charbonnel, N. (2023) Pathogen community composition and co-infection patterns in a wild community of rodents. BioRxiv, ver.4 peer-reviewed and recommended by Peer Community in Ecology. https://doi.org/10.1101/2020.02.09.940494 Andreazzi, C. S., Martinez-Vaquero, L. A., Winck, G. R., Cardoso, T. S., Teixeira, B. R., Xavier, S. C. C., Gentile, R., Jansen, A. M. & D'Andrea, P. S. (2023) Vegetation cover and biodiversity reduce parasite infection in wild hosts across ecological levels and scales. Ecography, 2023, e06579. | Pathogen community composition and co-infection patterns in a wild community of rodents | Jessica Lee Abbate, Maxime Galan, Maria Razzauti, Tarja Sironen, Liina Voutilainen, Heikki Henttonen, Patrick Gasqui, Jean-François Cosson, Nathalie Charbonnel | <p style="text-align: justify;">Rodents are major reservoirs of pathogens that can cause disease in humans and livestock. It is therefore important to know what pathogens naturally circulate in rodent populations, and to understand the factors tha... |  | Biodiversity, Coexistence, Community ecology, Eco-immunology & Immunity, Epidemiology, Host-parasite interactions, Population ecology, Species distributions | Francois Massol | 2020-02-11 12:42:28 | View | |
03 Jan 2024
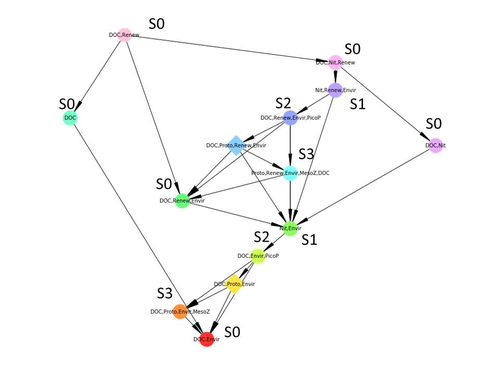
Diagnosis of planktonic trophic network dynamics with sharp qualitative changesCedric Gaucherel, Stolian Fayolle, Raphael Savelli, Olivier Philippine, Franck Pommereau, Christine Dupuy https://doi.org/10.1101/2023.06.29.547055A new approach to describe qualitative changes of complex trophic networksRecommended by Francis Raoul based on reviews by Tim Coulson and 1 anonymous reviewerModelling the temporal dynamics of trophic networks has been a key challenge for community ecologists for decades, especially when anthropogenic and natural forces drive changes in species composition, abundance, and interactions over time. So far, most modelling methods fail to incorporate the inherent complexity of such systems, and its variability, to adequately describe and predict temporal changes in the topology of trophic networks. Taking benefit from theoretical computer science advances, Gaucherel and colleagues (2024) propose a new methodological framework to tackle this challenge based on discrete-event Petri net methodology. To introduce the concept to naïve readers the authors provide a useful example using a simplistic predator-prey model. The core biological system of the article is a freshwater trophic network of western France in the Charente-Maritime marshes of the French Atlantic coast. A directed graph describing this system was constructed to incorporate different functional groups (phytoplankton, zooplankton, resources, microbes, and abiotic components of the environment) and their interactions. Rules and constraints were then defined to, respectively, represent physiochemical, biological, or ecological processes linking network components, and prevent the model from simulating unrealistic trajectories. Then the full range of possible trajectories of this mechanistic and qualitative model was computed. The model performed well enough to successfully predict a theoretical trajectory plus two trajectories of the trophic network observed in the field at two different stations, therefore validating the new methodology introduced here. The authors conclude their paper by presenting the power and drawbacks of such a new approach to qualitatively model trophic networks dynamics. Reference Cedric Gaucherel, Stolian Fayolle, Raphael Savelli, Olivier Philippine, Franck Pommereau, Christine Dupuy (2024) Diagnosis of planktonic trophic network dynamics with sharp qualitative changes. bioRxiv 2023.06.29.547055, ver. 2 peer-reviewed and recommended by Peer Community in Ecology. https://doi.org/10.1101/2023.06.29.547055 | Diagnosis of planktonic trophic network dynamics with sharp qualitative changes | Cedric Gaucherel, Stolian Fayolle, Raphael Savelli, Olivier Philippine, Franck Pommereau, Christine Dupuy | <p>Trophic interaction networks are notoriously difficult to understand and to diagnose (i.e., to identify contrasted network functioning regimes). Such ecological networks have many direct and indirect connections between species, and these conne... |  | Community ecology, Ecosystem functioning, Food webs, Freshwater ecology, Interaction networks, Microbial ecology & microbiology | Francis Raoul | Tim Coulson | 2023-07-03 10:42:34 | View |
29 Dec 2018
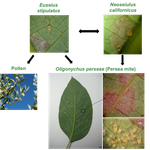
The return of the trophic chain: fundamental vs realized interactions in a simple arthropod food webInmaculada Torres-Campos, Sara Magalhães, Jordi Moya-Laraño, Marta Montserrat https://doi.org/10.1101/324178From deserts to avocado orchards - understanding realized trophic interactions in communitiesRecommended by Francis John Burdon based on reviews by Owen Petchey and 2 anonymous reviewersThe late eminent ecologist Gary Polis once stated that “most catalogued food-webs are oversimplified caricatures of actual communities” and are “grossly incomplete representations of communities in terms of both diversity and trophic connections.” Not content with that damning indictment, he went further by railing that “theorists are trying to explain phenomena that do not exist” [1]. The latter critique might have been push back for Robert May´s ground-breaking but ultimately flawed research on the relationship between food-web complexity and stability [2]. Polis was a brilliant ecologist, and his thinking was clearly influenced by his experiences researching desert food webs. Those food webs possess an uncommon combination of properties, such as frequent omnivory, cannibalism, and looping; high linkage density (L/S); and a nearly complete absence of apex consumers, since few species completely lack predators or parasites [3]. During my PhD studies, I was lucky enough to visit Joshua Tree National Park on the way to a conference in New England, and I could immediately see the problems posed by desert ecosystems. At the time, I was ruminating on the “harsh-benign” hypothesis [4], which predicts that the relative importance of abiotic and biotic forces should vary with changes in local environmental conditions (from harsh to benign). Specifically, in more “harsh” environments, abiotic factors should determine community composition whilst weakening the influence of biotic interactions. However, in the harsh desert environment I saw first-hand evidence that species interactions were not diminished; if anything, they were strengthened. Teddy-bear chollas possessed murderously sharp defenses to protect precious water, creosote bushes engaged in belowground “chemical warfare” (allelopathy) to deter potential competitors, and rampant cannibalism amongst scorpions drove temporal and spatial ontogenetic niche partitioning. Life in the desert was hard, but you couldn´t expect your competition to go easy on you. References [1] Polis, G. A. (1991). Complex trophic interactions in deserts: an empirical critique of food-web theory. The American Naturalist, 138(1), 123-155. doi: 10.1086/285208 | The return of the trophic chain: fundamental vs realized interactions in a simple arthropod food web | Inmaculada Torres-Campos, Sara Magalhães, Jordi Moya-Laraño, Marta Montserrat | <p>The mathematical theory describing small assemblages of interacting species (community modules or motifs) has proved to be essential in understanding the emergent properties of ecological communities. These models use differential equations to ... |  | Community ecology, Experimental ecology | Francis John Burdon | 2018-05-16 19:34:10 | View | |
30 Sep 2020
How citizen science could improve Species Distribution Models and their independent assessmentFlorence Matutini, Jacques Baudry, Guillaume Pain, Morgane Sineau, Josephine Pithon https://doi.org/10.1101/2020.06.02.129536Citizen science contributes to SDM validationRecommended by Francisco Lloret based on reviews by Maria Angeles Perez-Navarro and 1 anonymous reviewerCitizen science is becoming an important piece for the acquisition of scientific knowledge in the fields of natural sciences, and particularly in the inventory and monitoring of biodiversity (McKinley et al. 2017). The information generated with the collaboration of citizens has an evident importance in conservation, by providing information on the state of populations and habitats, helping in mitigation and restoration actions, and very importantly contributing to involve society in conservation (Brown and Williams 2019).
An obvious advantage of these initiatives is the ability to mobilize human resources on a large territorial scale and in the medium term, which would otherwise be difficult to finance. The resulting increasing information then can be processed with advanced computational techniques (Hochachka et al 2012; Kelling et al. 2015), thus improving our interpretation of the distribution of species. Specifically, the ability to obtain information on a large territorial scale can be integrated into studies based on Species Distribution Models SDMs. One of the common problems with SDMs is that they often work from species occurrences that have been opportunistically recorded, either by professionals or amateurs. A great challenge for data obtained from non-professional citizens, however, remains to ensure its standardization and quality (Kosmala et al. 2016). This requires a clear and effective design, solid volunteer training, and a high level of coordination that turns out to be complex (Brown and Williams 2019). Finally, it is essential to perform a quality validation following scientifically recognized standards, since they are often conditioned by errors and biases in obtaining information (Bird et al. 2014). There are two basic approaches to obtain the necessary data for this validation: getting it from an external source (external validation), or allocating a part of the database itself (internal validation or cross-validation) to this function. References [1] Bird TJ et al. (2014) Statistical solutions for error and bias in global citizen science datasets. Biological Conservation 173: 144-154. doi: 10.1016/j.biocon.2013.07.037 | How citizen science could improve Species Distribution Models and their independent assessment | Florence Matutini, Jacques Baudry, Guillaume Pain, Morgane Sineau, Josephine Pithon | <p>Species distribution models (SDM) have been increasingly developed in recent years but their validity is questioned. Their assessment can be improved by the use of independent data but this can be difficult to obtain and prohibitive to collect.... | Biodiversity, Biogeography, Conservation biology, Habitat selection, Spatial ecology, Metacommunities & Metapopulations, Species distributions, Statistical ecology | Francisco Lloret | 2020-06-03 09:36:34 | View | ||
06 Oct 2020
Implementing a rapid geographic range expansion - the role of behavior and habitat changesLogan CJ, McCune KB, Chen N, Lukas D http://corinalogan.com/Preregistrations/gxpopbehaviorhabitat.htmlThe role of behavior and habitat availability on species geographic expansionRecommended by Esther Sebastián González based on reviews by Caroline Marie Jeanne Yvonne Nieberding, Pizza Ka Yee Chow, Tim Parker and 1 anonymous reviewer based on reviews by Caroline Marie Jeanne Yvonne Nieberding, Pizza Ka Yee Chow, Tim Parker and 1 anonymous reviewer
Understanding the relative importance of species-specific traits and environmental factors in modulating species distributions is an intriguing question in ecology [1]. Both behavioral flexibility (i.e., the ability to change the behavior in changing circumstances) and habitat availability are known to influence the ability of a species to expand its geographic range [2,3]. However, the role of each factor is context and species dependent and more information is needed to understand how these two factors interact. In this pre-registration, Logan et al. [4] explain how they will use Great-tailed grackles (Quiscalus mexicanus), a species with a flexible behavior and a rapid geographic range expansion, to evaluate the relative role of habitat and behavior as drivers of the species’ expansion [4]. The authors present very clear hypotheses, predicted results and also include alternative predictions. The rationales for all the hypotheses are clearly stated, and the methodology (data and analyses plans) are described with detail. The large amount of information already collected by the authors for the studied species during previous projects warrants the success of this study. It is also remarkable that the authors will make all their data available in a public repository, and that the pre-registration in already stored in GitHub, supporting open access and reproducible science. I agree with the three reviewers of this pre-registration about its value and I think its quality has largely improved during the review process. Thus, I am happy to recommend it and I am looking forward to seeing the results. References [1] Gaston KJ. 2003. The structure and dynamics of geographic ranges. Oxford series in Ecology and Evolution. Oxford University Press, New York. [2] Sol D, Lefebvre L. 2000. Behavioural flexibility predicts invasion success in birds introduced to new zealand. Oikos. 90(3): 599–605. https://doi.org/10.1034/j.1600-0706.2000.900317.x [3] Hanski I, Gilpin M. 1991. Metapopulation dynamics: Brief history and conceptual domain. Biological journal of the Linnean Society. 42(1-2): 3–16. https://doi.org/10.1111/j.1095-8312.1991.tb00548.x [4] Logan CJ, McCune KB, Chen N, Lukas D. 2020. Implementing a rapid geographic range expansion - the role of behavior and habitat changes (http://corinalogan.com/Preregistrations/gxpopbehaviorhabitat.html) In principle acceptance by PCI Ecology of the version on 16 Dec 2021 https://github.com/corinalogan/grackles/blob/0fb956040a34986902a384a1d8355de65010effd/Files/Preregistrations/gxpopbehaviorhabitat.Rmd. | Implementing a rapid geographic range expansion - the role of behavior and habitat changes | Logan CJ, McCune KB, Chen N, Lukas D | <p>It is generally thought that behavioral flexibility, the ability to change behavior when circumstances change, plays an important role in the ability of a species to rapidly expand their geographic range (e.g., Lefebvre et al. (1997), Griffin a... | Behaviour & Ethology, Biological invasions, Dispersal & Migration, Foraging, Habitat selection, Human impact, Phenotypic plasticity, Preregistrations, Zoology | Esther Sebastián González | Anonymous, Caroline Marie Jeanne Yvonne Nieberding, Tim Parker | 2020-05-14 11:18:57 | View | |
03 Feb 2023

The role of climate change and niche shifts in divergent range dynamics of a sister-species pairJeremy Summers, Dieter Lukas, Corina J. Logan, Nancy Chen https://doi.org/10.32942/osf.io/879peDrivers of range expansion in a pair of sister grackle speciesRecommended by Esther Sebastián González based on reviews by 2 anonymous reviewers based on reviews by 2 anonymous reviewers
The spatial distribution of a species is driven by both biotic and abiotic factors that may change over time (Soberón & Nakamura, 2009; Paquette & Hargreaves, 2021). Therefore, species ranges are dynamic, especially in humanized landscapes where changes occur at high speeds (Sirén & Morelli, 2020). The distribution of many species is being reduced because of human impacts; however, some species are expanding their distributions, even over their niche (Lustenhouwer & Parker, 2022). One of the factors that may lead to a geographic niche expansion is behavioral flexibility (Mikhalevich et al., 2017), but the mechanisms determining range expansion through behavioral changes are not fully understood. The PCI Ecology study by Summers et al. (2023) uses a very large database on the current and historic distribution of two species of grackles that have shown different trends in their distribution. The great-tailed grackle has largely expanded its range over the 20th century, while the range of the boat-tailed grackle has remained very similar. They take advantage of this differential response in the distribution of the two species and run several analyses to test whether it was a change in habitat availability, in the realized niche, in habitat connectivity or in in the other traits or conditions that previously limited the species range, what is driving the observed distribution of the species. The study finds a change in the niche of great-tailed grackle, consistent with the high behavioral flexibility of the species. The two reviewers and I have seen a lot of value in this study because 1) it addresses a very timely question, especially in the current changing world; 2) it is a first step to better understanding if behavioral attributes may affect species’ ability to change their niche; 3) it contrasts the results using several complementary statistical analyses, reinforcing their conclusions; 4) it is based on the preregistration Logan et al (2021), and any deviations from it are carefully explained and justified in the text and 5) the limitations of the study have been carefully discussed. It remains to know if the boat-tailed grackle has more limited behavioral flexibility than the great-tailed grackle, further confirming the results of this study. Logan CJ, McCune KB, Chen N, Lukas D (2021) Implementing a rapid geographic range expansion - the role of behavior and habitat changes. http://corinalogan.com/Preregistrations/gxpopbehaviorhabitat.html Lustenhouwer N, Parker IM (2022) Beyond tracking climate: Niche shifts during native range expansion and their implications for novel invasions. Journal of Biogeography, 49, 1481–1493. https://doi.org/10.1111/jbi.14395 Mikhalevich I, Powell R, Logan C (2017) Is behavioural flexibility evidence of cognitive complexity? How evolution can inform comparative cognition. Interface Focus, 7, 20160121. https://doi.org/10.1098/rsfs.2016.0121 Paquette A, Hargreaves AL (2021) Biotic interactions are more often important at species’ warm versus cool range edges. Ecology Letters, 24, 2427–2438. https://doi.org/10.1111/ele.13864 Sirén APK, Morelli TL (2020) Interactive range-limit theory (iRLT): An extension for predicting range shifts. Journal of Animal Ecology, 89, 940–954. https://doi.org/10.1111/1365-2656.13150 Soberón J, Nakamura M (2009) Niches and distributional areas: Concepts, methods, and assumptions. Proceedings of the National Academy of Sciences, 106, 19644–19650. https://doi.org/10.1073/pnas.0901637106 Summers JT, Lukas D, Logan CJ, Chen N (2022) The role of climate change and niche shifts in divergent range dynamics of a sister-species pair. EcoEvoRxiv, ver. 3 peer-reviewed and recommended by Peer Community in Ecology. https://doi.org/10.32942/osf.io/879pe | The role of climate change and niche shifts in divergent range dynamics of a sister-species pair | Jeremy Summers, Dieter Lukas, Corina J. Logan, Nancy Chen | <p>---This is a POST-STUDY manuscript for the PREREGISTRATION, which received in principle acceptance in 2020 from Dr. Sebastián González (reviewed by Caroline Nieberding, Tim Parker, and Pizza Ka Yee Chow; <a href="https://doi.org/10.24072/pci.ec... |  | Behaviour & Ethology, Biogeography, Dispersal & Migration, Human impact, Landscape ecology, Preregistrations, Species distributions | Esther Sebastián González | 2022-05-26 20:07:33 | View | |
28 Aug 2023
Implementing a rapid geographic range expansion - the role of behavior changesLogan CJ, McCune KB, LeGrande-Rolls C, Marfori Z, Hubbard J, Lukas D https://doi.org/10.32942/X2N30JBehavioral changes in the rapid geographic expansion of the great-tailed grackleRecommended by Esther Sebastián González based on reviews by Francois-Xavier Dechaume-Moncharmont, Pizza Ka Yee Chow and 1 anonymous reviewer based on reviews by Francois-Xavier Dechaume-Moncharmont, Pizza Ka Yee Chow and 1 anonymous reviewer
While many species' populations are declining, primarily due to human-related impacts (McKnee et al., 2014), certain species have thrived by utilizing human-influenced environments, leading to their population expansion (Muñoz & Real, 2006). In this context, the capacity to adapt and modify behaviors in response to new surroundings is believed to play a crucial role in facilitating species' spread to novel areas (Duckworth & Badyaev, 2007). For example, an increase in innovative behaviors within recently established communities could aid in discovering previously untapped food resources, while a decrease in exploration might reduce the likelihood of encountering dangers in unfamiliar territories (e.g., Griffin et al., 2016). To investigate the contribution of these behaviors to rapid range expansions, it is essential to directly measure and compare behaviors in various populations of the species. The study conducted by Logan et al. (2023) aims to comprehend the role of behavioral changes in the range expansion of great-tailed grackles (Quiscalus mexicanus). To achieve this, the researchers compared the prevalence of specific behaviors at both the expansion's edge and its middle. Great-tailed grackles were chosen as an excellent model due to their behavioral adaptability, rapid geographic expansion, and their association with human-modified environments. The authors carried out a series of experiments in captivity using wild-caught individuals, following a detailed protocol. The study successfully identified differences in two of the studied behavioral traits: persistence (individuals participated in a larger proportion of trials) and flexibility variance (a component of the species' behavioral flexibility, indicating a higher chance that at least some individuals in the population could be more flexible). Notably, individuals at the edge of the population exhibited higher values of persistence and flexibility, suggesting that these behavioral traits might be contributing factors to the species' expansion. Overall, the study by Logan et al. (2023) is an excellent example of the importance of behavioral flexibility and other related behaviors in the process of species' range expansion and the significance of studying these behaviors across different populations to gain a better understanding of their role in the expansion process. Finally, it is important to underline that this study is part of a pre-registration that received an In Principle Recommendation in PCI Ecology (Sebastián-González 2020) where objectives, methodology, and expected results were described in detail. The authors have identified any deviation from the original pre-registration and thoroughly explained the reasons for their deviations, which were very clear. References Duckworth, R. A., & Badyaev, A. V. (2007). Coupling of dispersal and aggression facilitates the rapid range expansion of a passerine bird. Proceedings of the National Academy of Sciences, 104(38), 15017-15022. https://doi.org/10.1073/pnas.0706174104 Griffin, A.S., Guez, D., Federspiel, I., Diquelou, M., Lermite, F. (2016). Invading new environments: A mechanistic framework linking motor diversity and cognition to establishment success. Biological Invasions and Animal Behaviour, 26e46. https://doi.org/10.1017/CBO9781139939492.004 Logan, C. J., McCune, K., LeGrande-Rolls, C., Marfori, Z., Hubbard, J., Lukas, D. 2023. Implementing a rapid geographic range expansion - the role of behavior changes. EcoEvoRxiv, ver. 3 peer-reviewed and recommended by PCI Ecology. https://doi.org/10.32942/X2N30J McKee, J. K., Sciulli, P. W., Fooce, C. D., & Waite, T. A. (2004). Forecasting global biodiversity threats associated with human population growth. Biological Conservation, 115(1), 161-164. https://doi.org/10.1016/S0006-3207(03)00099-5 Muñoz, A. R., & Real, R. (2006). Assessing the potential range expansion of the exotic monk parakeet in Spain. Diversity and Distributions, 12(6), 656-665. https://doi.org/10.1111/j.1472-4642.2006.00272.x Sebastián González, E. (2020) The role of behavior and habitat availability on species geographic expansion. Peer Community in Ecology, 100062. https://doi.org/10.24072/pci.ecology.100062. Reviewers: Caroline Nieberding, Tim Parker, and Pizza Ka Yee Chow. | Implementing a rapid geographic range expansion - the role of behavior changes | Logan CJ, McCune KB, LeGrande-Rolls C, Marfori Z, Hubbard J, Lukas D | <p>It is generally thought that behavioral flexibility, the ability to change behavior when circumstances change, plays an important role in the ability of species to rapidly expand their geographic range. Great-tailed grackles (<em>Quiscalus mexi... | Behaviour & Ethology, Preregistrations, Zoology | Esther Sebastián González | 2023-04-12 11:00:42 | View | ||
18 Apr 2024

Insights on the effect of mega-carcass abundance on the population dynamics of a facultative scavenger predator and its preyMellina Sidous; Sarah Cubaynes; Olivier Gimenez; Nolwenn Drouet-Hoguet; Stephane Dray; Loic Bollache; Daphine Madhlamoto; Nobesuthu Adelaide Ngwenya; Herve Fritz; Marion Valeix https://doi.org/10.1101/2023.11.08.566247Unveiling the influence of carrion pulses on predator-prey dynamicsRecommended by Esther Sebastián González based on reviews by Eli Strauss and 1 anonymous reviewer based on reviews by Eli Strauss and 1 anonymous reviewer
Most, if not all, predators consume carrion in some circumstances (Sebastián-Gonzalez et al. 2023). Consequently, significant fluctuations in carrion availability can impact predator-prey dynamics by altering the ratio of carrion to live prey in the predators' diet (Roth 2003). Changes in carrion availability may lead to reduced predation when carrion is more abundant (hypo-predation) and intensified predation if predator populations surge in response to carrion influxes but subsequently face scarcity (hyper-predation), (Moleón et al. 2014, Mellard et al. 2021). However, this relationship between predation and scavenging is often challenging because of the lack of empirical data. | Insights on the effect of mega-carcass abundance on the population dynamics of a facultative scavenger predator and its prey | Mellina Sidous; Sarah Cubaynes; Olivier Gimenez; Nolwenn Drouet-Hoguet; Stephane Dray; Loic Bollache; Daphine Madhlamoto; Nobesuthu Adelaide Ngwenya; Herve Fritz; Marion Valeix | <p>The interplay between facultative scavenging and predation has gained interest in the last decade. The prevalence of scavenging induced by the availability of large carcasses may modify predator density or behaviour, potentially affecting prey.... |  | Community ecology | Esther Sebastián González | Eli Strauss | 2023-11-14 15:27:16 | View |
MANAGING BOARD
Julia Astegiano
Tim Coulson
Anna Eklof
Dominique Gravel
François Massol
Ben Phillips
Cyrille Violle










