
Water primerose (Ludwigia grandiflora subsp. hexapetala) auto- and allogamy: an ecological perspective
Late-acting self-incompatible system, preferential allogamy and delayed selfing in the heterostylous invasive populations of Ludwigia grandiflora subsp. hexapetala
Abstract
Recommendation: posted 28 March 2022, validated 05 April 2022
Vernay, A. (2022) Water primerose (Ludwigia grandiflora subsp. hexapetala) auto- and allogamy: an ecological perspective. Peer Community in Ecology, 100095. https://doi.org/10.24072/pci.ecology.100095
Recommendation
Invasive plant species are widely studied by the ecologist community, especially in wetlands. Indeed, alien plants are considered one of the major threats to wetland biodiversity (Reid et al., 2019). Ludwigia grandiflora subsp. hexapetala (Hook. & Arn.) G.L.Nesom & Kartesz, 2000 (Lgh) is one of them and has received particular attention for a long time (Hieda et al., 2020; Thouvenot, Haury, & Thiebaut, 2013). The ecology of this invasive species and its effect on its biotic and abiotic environment has been studied in previous works. Different processes were demonstrated to explain their invasibility such as allelopathic interference (Dandelot et al., 2008), resource competition (Gérard et al., 2014), and high phenotypic plasticity (Thouvenot, Haury, & Thiébaut, 2013), to cite a few of them. However, although vegetative reproduction is a well-known invasive process for alien plants like Lgh (Glover et al., 2015), the sexual reproduction of this species is still unclear and may help to understand the Lgh population dynamics.
Portillo Lemus et al. (2021) showed that two floral morphs of Lgh co-exist in natura, involving self-compatibility for short-styled phenotype and self-incompatibility for long-styled phenotype processes. This new article (Portillo Lemus et al., 2022) goes further and details the underlying mechanisms of the sexual reproduction of the two floral morphs.
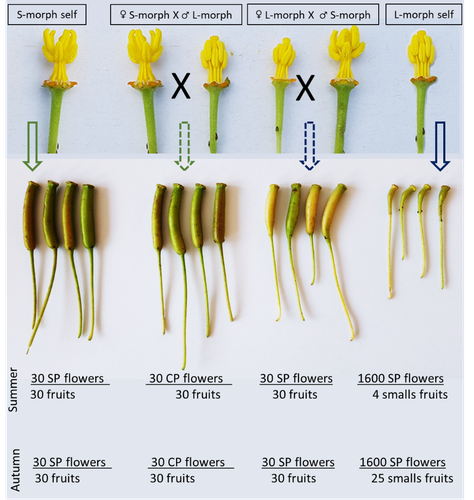
Complementing their previous study, the authors have described a late self-incompatible process associated with the long-styled morph, which authorized a small proportion of autogamy. Although this represents a small fraction of the L-morph reproduction, it may have a considerable impact on the L-morph population dynamics. Indeed, authors report that “floral morphs are mostly found in allopatric monomorphic populations (i.e., exclusively S-morph or exclusively L-morph populations)” with a large proportion of L-morph populations compared to S-morph populations in the field. It may seem counterintuitive as L-morph mainly relies on cross-fecundation.
Results show that L-morph autogamy mainly occurs in the fall, late in the reproduction season. Therefore, the reproduction may be ensured if no exogenous pollen reaches the stigma of L-morph individuals. It partly explains the large proportion of L-morph populations in the field.
Beyond the description of late-acting self-incompatibility, which makes the Onagraceae a third family of Myrtales with this reproductive adaptation, the study raises several ecological questions linked to the results presented in the article. First, it seems that even if autogamy is possible, Lgh would favour allogamy, even in S-morph, through the faster development of pollen tubes from other individuals. This may confer an adaptative and evolutive advantage for the Lgh, increasing its invasive potential. The article shows this faster pollen tube development in S-morph but does not test the evolutive consequences. It is an interesting perspective for future research. It would also be interesting to describe cellular processes which recognize and then influence the speed of the pollen tube. Second, the importance of sexual reproduction vs vegetative reproduction would also provide information on the benefits of sexual dimorphism within populations. For instance, how fruit production increases the dispersal potential of Lgh would help to understand Lgh population dynamics and to propose adapted management practices (Delbart et al., 2013; Meisler, 2009).
To conclude, the study proposes a morphological, reproductive and physiological description of the Lgh sexual reproduction process. However, underlying ecological questions are well included in the article and the ecophysiological results enlighten some questions about the role of sexual reproduction in the invasiveness of Lgh. I advise the reader to pay attention to the reviewers’ comments; the debates were very constructive and, thanks to the great collaboration with the authorship, lead to an interesting paper about Lgh reproduction and with promising perspectives in ecology and invasion ecology.
References
Dandelot S, Robles C, Pech N, Cazaubon A, Verlaque R (2008) Allelopathic potential of two invasive alien Ludwigia spp. Aquatic Botany, 88, 311–316. https://doi.org/10.1016/j.aquabot.2007.12.004
Delbart E, Mahy G, Monty A (2013) Efficacité des méthodes de lutte contre le développement de cinq espèces de plantes invasives amphibies : Crassula helmsii, Hydrocotyle ranunculoides, Ludwigia grandiflora, Ludwigia peploides et Myriophyllum aquaticum (synthèse bibliographique). BASE, 17, 87–102. https://popups.uliege.be/1780-4507/index.php?id=9586
Gérard J, Brion N, Triest L (2014) Effect of water column phosphorus reduction on competitive outcome and traits of Ludwigia grandiflora and L. peploides, invasive species in Europe. Aquatic Invasions, 9, 157–166. https://doi.org/10.3391/ai.2014.9.2.04
Glover R, Drenovsky RE, Futrell CJ, Grewell BJ (2015) Clonal integration in Ludwigia hexapetala under different light regimes. Aquatic Botany, 122, 40–46. https://doi.org/10.1016/j.aquabot.2015.01.004
Hieda S, Kaneko Y, Nakagawa M, Noma N (2020) Ludwigia grandiflora (Michx.) Greuter & Burdet subsp. hexapetala (Hook. & Arn.) G. L. Nesom & Kartesz, an Invasive Aquatic Plant in Lake Biwa, the Largest Lake in Japan. Acta Phytotaxonomica et Geobotanica, 71, 65–71. https://doi.org/10.18942/apg.201911
Meisler J (2009) Controlling Ludwigia hexaplata in Northern California. Wetland Science and Practice, 26, 15–19. https://doi.org/10.1672/055.026.0404
Portillo Lemus LO, Harang M, Bozec M, Haury J, Stoeckel S, Barloy D (2022) Late-acting self-incompatible system, preferential allogamy and delayed selfing in the heteromorphic invasive populations of Ludwigia grandiflora subsp. hexapetala. bioRxiv, 2021.07.15.452457, ver. 4 peer-reviewed and recommended by Peer Community in Ecology. https://doi.org/10.1101/2021.07.15.452457
Portillo Lemus LO, Bozec M, Harang M, Coudreuse J, Haury J, Stoeckel S, Barloy D (2021) Self-incompatibility limits sexual reproduction rather than environmental conditions in an invasive water primrose. Plant-Environment Interactions, 2, 74–86. https://doi.org/10.1002/pei3.10042
Reid AJ, Carlson AK, Creed IF, Eliason EJ, Gell PA, Johnson PTJ, Kidd KA, MacCormack TJ, Olden JD, Ormerod SJ, Smol JP, Taylor WW, Tockner K, Vermaire JC, Dudgeon D, Cooke SJ (2019) Emerging threats and persistent conservation challenges for freshwater biodiversity. Biological Reviews, 94, 849–873. https://doi.org/10.1111/brv.12480
Thouvenot L, Haury J, Thiebaut G (2013) A success story: water primroses, aquatic plant pests. Aquatic Conservation: Marine and Freshwater Ecosystems, 23, 790–803. https://doi.org/10.1002/aqc.2387
Thouvenot L, Haury J, Thiébaut G (2013) Seasonal plasticity of Ludwigia grandiflora under light and water depth gradients: An outdoor mesocosm experiment. Flora - Morphology, Distribution, Functional Ecology of Plants, 208, 430–437. https://doi.org/10.1016/j.flora.2013.07.004
The recommender in charge of the evaluation of the article and the reviewers declared that they have no conflict of interest (as defined in the code of conduct of PCI) with the authors or with the content of the article. The authors declared that they comply with the PCI rule of having no financial conflicts of interest in relation to the content of the article.
Evaluation round #3
DOI or URL of the preprint: https://doi.org/10.1101/2021.07.15.452457
Version of the preprint: 3
Author's Reply, 14 Mar 2022
Decision by Antoine Vernay , posted 07 Mar 2022
, posted 07 Mar 2022
Dear authors,
This second round of your manuscript is now completed. The reviewer made a great job on this new version and encouraged you to improve it with small changes. Please consider the minor revisions asked by the reviewer. I think we are now close to the final version of your manuscript. If you have any questions, please contact me.
Best regards,
Download recommender's annotationsReviewed by Emiliano Mora-Carrera, 04 Mar 2022
This is the second round of revisions of this manuscript. The main results are that: both floral morphs of Lgh do not have differences in pollen and stigma shape; there is evidence of LSI acting in Lgh, but only on the L-morph; outcrossed pollen is more successful than self-pollen at fertilizing the ovules due to faster pollen tube elongation. The authors use these results to explain the puzzling observation that the L-morph floral is more common in invasive populations despite being SI. This is the first detailed report of LSI in Ludwigia and confirms the presence of LSI in a third family within the Myrtales order.
In my opinion, the manuscript, specifically the Introduction and Discussion, has improved substantially by implementing the comments from previous reviewers. As indicated in previous revisions, the methods and results are thorough and sound, and deserve to be published. However, some minor changes in the manuscript need to be made. For instance, some typos (that I indicate above) need to be corrected. I made several suggestions throughout the manuscript in the hope that they increase its readability. In my opinion, most of the suggestions can be implemented quite easily, except for changes in the 2nd part of the Discussion. These of course are suggestions and their suitability should be discussed between the authors and the editor.
Having said the above, I suggest that this paper should be recommended for publication in PCI Ecology, provided the authors make the specific changes above.
--------------------------------------------------------
Abstract
L28: Use 'among' or 'between', but not both.
L31: Maybe change "... questions on the distribution of this breeding system ..." for "... contributes a case of LSI in an additional family within the order Myrtales.", or something similar.
Introduction
L47: Change 'promotes' for 'favors'. SI does not actively promote outcrossing (as in the case of heterostyly) but favors outcrossing.
L49: Eliminate "and fertilization in particular combinations of parents".
L53: Change "diversity" for "variation".
L55: Eliminate "Characterizing the type of SI individuals develop in a species by".
L56: Eliminate "first" or "essential". Keeping both is unnesessary.
L64-66: This sentence could be eliminated or moved to the first sentence of the paragraph. That way the last sentence of the first paragraph would be related to SI, giving a natural flow to the first sentence of the 2nd paragraph.
L72: Eliminate "... , or a continuous variation of floral morphologies not correlated with compatibility.".
L84: Change "occuring" to "it occurs".
L112: It would be good to also introduce and explain the other morph. For example "Conversely, in the S-morph ..."
L119-120: Reduce "... successful invasive species may be mostly composed of individuals able to reproduce using self-fertilization when mating" to "... succesful invasive species are the ones capable of self-fertilization".
Methods
L169: Eliminate "usually"
L170: Change 'Opale' to 'Opedal'.
Results
L271-278: Please provide the average herkogamy (+- SD).
Discussion:
368: Eliminate the "from" in the natural populations.
385: Change "present" to "displays".
387-389: Change 'heterostylous' to 'heteromorphic'.
L401: It is unclear what the authors mean when saying "genetic ancestrality between individuals may be limited". I think the sentences can be understood without it. IF this information is important, then the authors need to explain in MS why this is important. One or two sentences should be enough.
L384-421: In general this section needs a bit more of work. It feels like the connection between LSI and its presence in HetSI is not clear and the idea goes back and forth, specially in the first three paragraph of the section.
L409: Authors mention 'GSI system' that has not been specified anywhere else in the manuscript. I assume this is gametophytic SI. If so just state it.
L411: "..., most of the self-pollen" is imprecise. If you have the number (i.e., %90 of self-pollen tubes) it would be better to give specific information.
L416: Eliminate 'other'.
L420-421: Eliminate this sentence or fuse it with the previous. Otherwise sound like repettion.
425: Eliminate "at a low, stable rate". It is distracting in this sentence and it is imprecise. 'low' is a relative term, and is better used when comparing two different observations (e.g., lower than ... or higher than ...). A similar thing happen when using "stable'.
L426: Eliminate "for some" and change "enabling" to "enables".
L425-428: This sentence has two main clauses (enables self-fertilization AND is present in multiple angiosperms) and is a bit hard to follow. I suggest the authors to split this sentence in two.
L430: I suggest to get rid of 'preferentially'. I think the sentence can be understood without it.
L431: Eliminate "some".
L459: Maybe add a sentence acknowledging that inbreeding depression could counteract the effect of selfing in local regeneration. A suggestion could be "Provided that inbreeding depression would not affect at later stages life cycle."
L460: Maybe change 'regenerate' with 'establish'.
L467: Change "..., better fitting Baker hypotheses." for ", providing support for Baker's Law.". Or if authors decide to keep it needs to be changed to "Baker's hypothesis".
L471: Red 'e' in where.
L473-475: This sentence is hard to follow. Specifically, "... invasive populations worldwide in different ecological contexts ...". I suggest to get rid of everything after 'invasive populations'. Or break the whole sentence in two.
Fig2: Put '(b)' on the top-right corner for consistency with the other images.
Evaluation round #2
DOI or URL of the preprint: 10.1101/2021.07.15.452457
Version of the preprint: 2
Author's Reply, 04 Feb 2022
Decision by Antoine Vernay , posted 29 Nov 2021
, posted 29 Nov 2021
Dear authors,
I carefully read your rebuttal letter about my first decision and I may make a decision "in hurry", as you suggested, therefore I asked the two previous reviewers to read again your reply. One reviewer declined the invitation to review your manuscript so I asked a third new one to do it. They now completed their reviews.
After reading their different comments, I think that the manuscript deserves some major changes again, especially because we need to read the entire manuscript (and not only your rebuttal letter) with your edits relative to the first round of review, and now to the second. My main concern is about the use of "heterostyly" which can be misleading as a reviewer wrote. However, reviewers made several constructive comments to improve the manuscript.
In your next version, please reply to all comments from all reviewers. You wrote your disagreements with some comments but consider reformulate when your argumentation seemed to not convince everybody. Reviewers most often, suggest some ways to reply to their comments. I can ensure you that the reviewers and I, have spent considerable time on your manuscript and we debate a lot about it. We felt that all the comments were appropriate and deserve improvements in the text. You mentioned that semantic can be easily changed, which is certainly true, but that will change the message of your paper, therefore I thought it was not trivial change.
I hope you will accept to edit your manuscript accordingly. I will then make a new decision about it.
Best regards
Download recommender's annotationsReviewed by anonymous reviewer 1, 27 Nov 2021
I find that my suggestions for improvement have been mostly incorporated into the revised paper; as I declared in the previous round, I consider the topic of this paper adequate and interesting to be published.
Finally, (as the authors exposed) further work with this species will be necessary to really understand the role of self-compatibility (S-morph), self-incompatibility (L-morph) and vegetative reproduction in the evolution and stability of both morphs in natural and invasive populations.
https://doi.org/10.24072/pci.ecology.100330.rev21Reviewed by Juan Arroyo, 18 Nov 2021
Dear PCI Ecology recommenders,
After reading the rebuttal letter by LO Portillo Lemus et al to my former review of their manuscript “Late-acting self-incompatible system, preferential allogamy and delayed selfing in the heterostylous invasive populations of Ludwigia grandiflora subsp. hexapetala” I consider that some of the most critical concerns I raised are still unresolved in their letter.
First, I would like to apologize if they feel that my review provoked a rude and hurried (their words) rejection or, more properly, an initial lack of recommendation. It was not my aim such a rudeness nor a rapid review. I read the manuscript several times and went to the relevant literature around the topic, including former published (that is, open to public scrutiny) work by the authors. It is my regular way to review.
Yes indeed, I consider the topic interesting of course. I have some doubts about its fitting to the scope of PCI Ecology, but this is subject to opinion which Recommenders and Managing Board should resolve, of course. My concerns were not about the semantics or the terms, and it is not a matter of being botanist, ecologist, or evolutionist. If I was very detailed in my report it was because I usually do that. I am or have been editor in other journals and know how frustrating can be a rejection based only in a short paragraph by an “established” reviewer. Although I have met similar cases as an editor, it is not common at all to re-submit the same manuscript after clear rejection. They have not corrected even those items where they offer a solution to concerns raised by at least one of the reviewers. It would have been good if at least these were addressed in a new version. This means that they prefer to put all time and effort in discussing the validity of the manuscript on very general grounds and opinions about the review process, which I think it is not the right place to do.
My main concerns were about the sampling design (how to know they were sampling different genotypes within a single highly clonal species is critical for this kind of study) and about stamen measurements, which are lacking and are necessary to determine heterostyly. If they do not want to speak about heterostyly, it is very easy to solve, just avoid it, from the very starting point: the title. This proper sampling of different genets is not addressed at all in their rebuttal letter. In fact, they answer to one of my concerns about this issue:
“Lines 169-179. The numbers of samples in this paragraph (which are large indeed) does not refers to how many individual plants (genets) and this is critical.
Answer: why it is critical knowing of randomly-sampled individuals in populations of thousands of plants are clones (ramets) or genets to understand their self-(in)compatiblity and if their selfed and outcrossed seeds are viable?”
Even if they aim to address in their study only incompatibility system, it is completely necessary to work with different genotypes. Incompatibility types, groups or morphs, if they prefer, are genetically based, thus the same genotype belong always to the same group. Since this is a problem of the design, I do not see how is possible to solve without a new sampling, or much easier, with a genetic screening of the plants used just to determine they are not clones. Random sampling of plants is fine for annual or perennial plants with no vegetative reproduction, simply by sampling a few meters apart; but this does not seem to be the case.
Just to be positive about the destiny of the manuscript, I prefer not to participate in the recommendation process further. I still think that there is an interesting point in their study (late acting self-incompatibility, in which I am not an expert). Finally, I have no objections, again, to accept a new case of heterostyly, when properly measured and analyzed. There are some recent cases in the literature which authors can check. This new case would much help in explaining the evolution of this breeding system, which usually provokes an outcrossing mating system in most of populations bearing it. I sincerely wish the authors are successful in their pursuits.
Juan Arroyo
https://doi.org/10.24072/pci.ecology.100330.rev22
Reviewed by Emiliano Mora-Carrera, 23 Nov 2021
In this manuscript, the authors make a comprehensive description of the self-incompatibility system in Ludwigia grandiflora. The main results of the manuscripts are that Ldh has two floral morphs that differ in the length of the style and that these floral morphs have differences in SI system. Specifically, the L-morph has a late SI system, whereas the S-morph is self-compatible. The manuscript has interesting results based on laboratory work and fieldwork, which are noteworthy and deserve to be published. However, in its current form, the manuscript has some important inaccuracies, and some changes need to be made. Mostly, I think there are some misuses of terms that could create confusion in the literature pertaining to the evolution of floral polymorphisms. If these inaccuracies are addressed, the manuscript has the potential to be recommended.
I went through the manuscript (first) and then through the authors' response to the comments of the Reviewers. I, however, see that despite the thorough response to the previous Reviewer's comments, no changes were made in the original manuscript (or at least not in the one that was given to review [https://doi.org/10.1101/2021.07.15.452457]).
I attempt to provide constructive criticism, as aked by the authors, in the hope that the authors reconsider and incorporate the comments of previous Reviewers that could improve ths manuscript. I will provide comments on the response from the authors (first) and then give specific comments on the current manuscript as it is now.
Specifically, I agree that Ludwigia grandiflora does not have heterostyly. Although there is a difference in the style length among the floral morphs (supported by statistical analyses in this manuscript), this floral system may be considered a style dimorphic species (with approach and reverse herkogamy). This is because the distinctive character of heterostyly is not the difference in style length alone, but the case of reverse herkogamy. Therefore, differences in anther position and the measure of herkogamy should also be reported (as pointed out by Reviewer 2) if one is to study heterostyly.
In their response to Reviewer 1 (from now on R1), the authors indicate that the main aim of the manuscript is to identify the mating system of Ldh and not about invasion ecology. I agree with this. However, the authors stress invasion too much in the Introduction that may mislead the reader into thinking that the paper is about invasion ecology (which also happened to me). I would temper the use of invasion in the Introduction keep it in the Discussion section. In fact, the observation that the SI morph is the most common in invasive populations is intriguing that could be exploited in the Discussion rather than in the Introduction.
Given that a great deal of the manuscript is based on the idea of having different floral morphs, I disagree with the authors in the part that "we don't interest in heteromorphy and floral biometry" and in "Whatever, whether or not this plant is heteromorphic is not the purpose of this current manuscript and definitely doesn't matter to study.". The authors say, "If specialists can demonstrate this is not heteromorphy or something else, we would be interested to read the demonstration and explanation, out of an argument of authority about a reference that reviewed ... ". My assessment of the paper (and the reviews) is not that Ldh does not have heteromorphy (which it has). Still, this heteromorphy (i.e., floral dimorphism) is NOT heterostyly. In fact, I don't believe that R1 and R2 disagree in that there are two floral morphs. They, however, disagree with the misuse of the term heterostyly. Heterostyly has a precise definition (Barrett 2019). I think that the authors demonstrate, very convincingly, that the flowers of Ldh are dimorphic, and thus, the authors can keep their use of L- and S-morph if they define them as approach and reverse herkogamy, but not as heterostylous. As indicated by the authors, the elimination of the term 'heterostyly' will not change the results; rather, it will keep consistency in the literature of heterostyly. Many problems in the evolution of floral polymorphisms occur due to misuse of these terms. In fact, one part of the Discussion could address whether Ldh is heterostylous or not. Moreover, one of the interesting results is that different floral morphs are associated with different SI systems, and if this is an original observation from this manuscript, the authors should exploit this in the Discussion.
I agree with R2 in that the possibility of vegetative reproduction should be at least acknowledged as an explanation of the fact that the SI morph (L-morph) is the most common in invasive populations. Authors do not need to make a whole discussion about it but only indicate that. Maybe supporting information from related taxa could be helpful.
In general, it would be valuable if the authors acknowledge the limitation of their study, in Discussion, and propose experiments that could fill the gaps in our knowledge regarding the reproduction Ldh and the role of reproduction in invasive population of Ldh.
--------------------------------------------------------------------------
Line 50: Get rid of "and beyond, and understand their phylogeny and evolution" since it adds nothing to the sentence and it is unclear. For instance, what is "beyond" angiosperms? It is unclear if the part that says 'their phylogeny' refers to angiosperms or to SI systems. In either case, SI is not essential to understanding the phylogeny of angiosperms. Moreover, one is interested in the 'phylogenetic distribution' of SI, not its phylogeny. I understand that the authors do not want to debate 'semantics' and 'terminology,' but some level of consistency should be kept for the scientific literature in order to avoid future confusion.
Line 42: Mixed mating system is misused here and in several parts of the manuscript (Lines: 6, 42, 330, 419, and 423). Although there seem to be potential differences in the mating system among the population (and maybe even within), the mating system requires specific sets of experiments (pollen flow among individuals, estimation of outcrossing rates, etc.). Mixed mating systems are defined as outcrossing rates between 0.2 and 0.8 (Goodwillie et al., 2005).
Line 58: I think that the use of 'peripatric' is very specific in this part of the manuscript, so I would not use it here.
Line 60: The authors already defined self-incompatibility as SI in the first paragraph. Use SI consistently throughout the manuscript, e.g., Line 359 and 363and so on (except when one starts a sentence).
Line 66: The authors define HetSI but then never use this acronym again. I would eliminate this acronym.
Line 69: The part "spatial distancing of the anthers and stigma in the 3D architecture of a flower" says nothing about heterostyly. By eliminating the introduction about heterostyly, the authors can get rid of this part.
Line 73: add a comma after 'i.e.' as is done in other parts of the ms.
Line 87: It is unclear to me why the use of 'literally' is important here.
Line 91: Ludwigia needs to be italicized.
Line 93: Change "Water primrose ..." to "The water Primrose ..."
Line 95: It would be great to know where does Ldh comes from (place of origin) and where it is invasive.
Line 99: This is where the confusion is generated. Ldh is already defined as heterostylous in the manuscript based on Portillo-Lemus et al. (2021) when in fact, this has not been proven (see my comment above). In fact, the term used in that paper is "heteromorphic reproductive system, " which is correct. Thus, I would suggest changing heterostyly to floral heteromorphism in all sections when discussing Ldh (e.g., Lines 346, 348, and 351 in discussion). Here the authors could define more clearly what they indicate as S- and L-morph.
Line 135: Eliminate "... compared the results we obtained with other species, especially from the Myrtales order to ... ". the comparison is irrelevant for the sentence.
Methods:
Line 215: Add space between the two paragraphs.
Results:
Line 252: Whenever p-value =<10-15 just state p-value <0.001. If it has 10, 13, or 15 zeroes, it makes no difference.
LIne 258: "... and whatever the pollen origin." could be changed to "... independent of the pollen origin".
Line 324: There seems to be a dash above a dot. Possibly due to a track change in Word.
Discussion:
Line 330: The term of mixed mating system is misused. Mixed mating indicates that outcrossing rates are between 0.2 to 0.8 (Goodwillie et al., 2006).
Line 335: The use of 'literally' is unnecessary.
Line 340: Change 'all self-pollens' to 'all self-pollen grains'.
Line 370: Vochysia should be in italics.
Line 377-385: It would be good if the authors point out the necessary experiments to determine 'reproductive assurance' in Ldh. For example, bagging experiments.
Table S1: In 3rd column it says 'fruitless' for some L-morph populations but then the fruit set is presented in the following columns which is contradictory information.
Figure S1: There are two 'S-morph self' treatments. I wonder if one of them is meant to be 'L-morph self'.
https://doi.org/10.24072/pci.ecology.100330.rev23Evaluation round #1
DOI or URL of the preprint: https://doi.org/10.1101/2021.07.15.452457
Version of the preprint: 1
Author's Reply, 25 Oct 2021
Dear Editorial board of PCI Ecology,
We would like first to thank you for your time and works on our manuscript. Please find attached our decision contestation and our answer point-by-point to reviewers and recommender.
We are still puzzled by the reasons that resulted to rudely and definitely reject our manuscript, while both reviewers acknowledged the interest of our study and the quality of our work. We would like to emphasis our will to continue the reviewing process in PCI Ecology, included with the two reviewers if they still agree to, or with others.
We thus request that our manuscript being still reviewed for recommendation in PCI Ecology or Evolutionary Biology. We are definitely convinced of the interest of our study for the scientific communities working on invasive plants, Onagraceae and Lythraceae, reproductive modes and self-incompatible system in plants, and for researchers and managers dealing with Ludwigia grandiflora subsp. hexapetala, one of the major invasive plants worldwide, currently colonizing France and European countries but also Japan and north America.
In the hope of a favourable decision in that direction, we will look forward to your answer in the next weeks.
Sincerely, the authors
Decision by Antoine Vernay , posted 22 Sep 2021
, posted 22 Sep 2021
Dear Dominique and colleagues,
First of all, I would like to apologize for this late decision, summer vacations slowed the reviewing process. Two referees were assigned to your manuscript, and in my opinion, read and commented on your paper very carefully.
Unfortunately, I have decided to not consider your manuscript for recommendation by PCI Ecology for two main reasons:
- As mentioned by referees, I agree that if reproduction is a key aspect of the invasion process, your experiment was not designed to fully address this question and did not bring clear evidence of the role of Ludwigia heterostyly in its invasive abilities. Your paper may more fit in a biology or physiology journal.
- the Ludwigia heterostyly is not consensual in the botanist community. The Onagraceae family does not even have any heterostyly species for some authors. I agree with the referees that this fundamental aspect of your study is not clearly demonstrated. Referees also highlighted several (maybe too many) inaccuracies in your introduction in the different comparisons with others studies you made among the manuscript.
Referees wrote several other concerns, suggestions, and advice to improve the paper but I think the manuscript needs too many modifications and reconsideration to be recommended even with major revisions.
I attach to this email the reviews of the two referees and I hope it can help you to improve your paper. Thanks for submitting your manuscript to PCI Ecology and I hope that, despite this rejection, you will consider PCI Ecology again for future submissions.
Best regards,
NB from the managing board of PCI Ecology: a procedure of appeal started the 25th of October 2021
Download recommender's annotationsReviewed by Juan Arroyo, 20 Sep 2021
Review of the manuscript
Late-acting self-incompatible system, preferential allogamy and delayed selfing in the heterostylous invasive populations of Ludwigia grandiflora subsp. hexapetala
by
Luis O. PORTILLO LEMUS, Marilyne HARANG, Michel BOZEC, Jacques HAURY, Solenn STOECKEL, Dominique BARLOY
Submitted to Peer Community in Ecology as https://doi.org/10.1101/2021.07.15.452457
This is an original study which deals with ascertaining biological traits (morphological, reproductive, genetic) of a species and their putative relationship with invasion ecology of the species. In short, the authors use their data on flower morphological variation in discrete style morphs, incompatibility systems, and reproductive output to infer processes on invasion ecology of the species invasion ecology. Thus, the whole study relies on an adequate description of morphs. I have several kinds of general comments on the suitability of this manuscript before the decision of PCI Ecology about its possible recommendation. Finally, I will provide some specific comments in the hope they will help in improving the manuscript.
About the topic
Whereas the topic is of interest to the public with a concern about biological invasions, I think that the information provided is not strictly and directly related with the process of invasion of the species. The biological traits reported are of course on potential implication on the invasion capacity of the species, but the authors are not strictly testing hypotheses about invasion, nor at local nor at global scales. Instead, the information provided is valuable in the context of botanical and plant biology literature. This information is about morphological, reproductive, embryological, and physiological aspects but by no means ecological ones, thus it is not possible to answer ecological hypotheses in a straightforward manner. In consequence, most of the Discussion is highly speculative.
About the data
Here there is my strongest concern about the manuscript. I mean that most of the study and the manuscript uses the concept of reciprocal style morphs and heteromorphic incompatibility just to support its significance for explaining a pattern of invasion. I must say that the data provided do not clearly demonstrate the existence of such morphs. This tenet is based in lines 98-102 and 108-112 of the Introduction, which is based in former studies by the authors and others (line 110: Hieda et al. 2020; Portillo-Lemus et al. 2021). I have checked carefully the information provided by these references and I do not think it demonstrates unequivocally the existence of heteromorphic incompatibility or even the existence of morphs. The reviewed manuscript neither does that. The study by Hieda et al. 2020 does not mention style morphs at all; may be the authors of the current manuscript infer the information from the morphological information provided by those authors, but it is unclear. Most importantly, the reviewed manuscript (and the former one by Portillo-Lemus et al 2021 Plant-Environment Interactions DOI: 10.1002/pei3.10042) lacks critical points in the design:
1. You need to take random samples of individuals (genets) where to pick flowers to take proper floral measurements. Whereas floral measurements for style length and stigma height are apparently well performed, I do not see the equivalent measurements for stamens. Critically, we do not know if separate genets were properly sampled in a species and populations where vegetative reproduction is so important.
2. In this context it is very surprising that former literature on systematics, reproductive biology and morphology on Onagraceae and Ludwigia, did not report heterostyly and heteromorphic incompatibility in the family and genus, as it was not reported in the several available surveys on heterostyly presence across angiosperms. It should be taken into account that some of the most remarkable papers were written by well-known scholars on the topic (particulalry PH Raven and RH Eyde, some of them cited by the authors). Of course, it is always possible to find new cases of heterostyly, but they should be properly documented. Just as a noteworthy mention by Peter H. Raven (1979) A survey of reproductive biology in Onagraceae, New Zealand Journal of Botany, 17:4, 575-593, DOI: 10.1080/0028825X.1979.10432572 with abundant information on Ludwigia which made clear that heterostyly was specifically searched:
"No species is known to be modally pollinated by bats, by beetles, or by wind, and none has apomixis involving seeds, nor heterostyly "
"Although it is frequent in the related family Lythraceae, heterostyly is unknown in Onagraceae."
Obviously discovering of hetersotyly in Onagraceae for the first time, as recognized by authors, would be a very interesting novelty, but should be properly supported by the data.
3. Heterostyly, and related style polymorphisms is, by definition, a population trait, because it works by disassortative mating, a negative frequency dependence mechanism. Of course, there are many reports of populations of heterostylous species that are otherwise monomorphic, just due to loss of one of the morphs or to colonization of only morph, which should have an alternative mode of reproduction (either vegetative, selfing, or intramorph compatibility). However, in all these cases, the species show some dimorphic source populations. These have not been reported by the authors and either in the former paper for the species under study by Portillo-Lemus et al 2021 Plant-Environment Interactions DOI: 10.1002/pei3.10042. Even that it is mentioned that 75% of the populations are composed by only L-morph plants, and that others are composed by the two morphs, the data provided do not accomplish requisites for proper morph sampling, nor there are data from native populations were both morphs should obviously exist.
4. Therefore, the pattern shown of a self-compatible (SC) morph and a self-incompatible (SI) morph in separate populations, if they really exist, could be explained by alternative hypotheses, for instance, the two “morphs” could be two lineages or species related but distinctly different given the difficult taxonomy of the species and the different ploidy levels in the group having different reproductive modes. The shift from SI to SC has been frequently reported and it is a very interesting research avenue according to cited Baker’s law, but it would need detailed phylogeographical and phylogenetic studies. Independently, it is very possible that the authors have sampled very few genotypes, or even only one, per population if vegetative reproduction is very strong. Again, this is a very relevant issue for invasion ecology which would need appropriate use of genetic markers to determine the genets/ramets being sampled. I recommend the authors explore these possibilities, and others, in depth with proper data and, if they are convinced that the species is truly heterostylous, gather the appropriate morphometric data for that.
5. The figures provided by the authors about flower measurements seem to show two different style length morphs (but see issues about sampling design: the flowers selected where different “morphs” a priori), but stamen length of morphs is not shown, which is needed for assessment of heterostyly. Besides, other factors such as style developmental stage across flower life span should also be considered.
Specific comments
Line 70. The species in Barranco et al. 2019 is not heterostylous, but stigma height dimorphic, although it is related with heterostyly, it has not reciprocal herkogamy as claimed by the authors.
Lines 85-87. Many of the Narcissus species included in Barrett et al. 2004 are not heterostylous, but stigma height dimorphic species, as it is the species studied by Simon-Porcar et al 2015. The species studied by Medrano et al 2012 is monomorphic.
Line 91. Ludwigia should be in italics
Line 92. were should be was
Line 121 and thereafter. What the authors report is information on breeding system or incompatibility system, nor mating system. This is a population trait which depicts what plant mates with what other plant. See Neal & Anderson Plant Syst. Evol. 250: 173–185 (2005) DOI 10.1007/s00606-004-0229-9 for clarification about these terms.
Line 125. Pollen and stigma dimorphism have never been reported as characteristic of homomorphic SI; even more, these differences cannot be taken as distinct features to separate homomorphic and heteromorphic SI systems as there are heteromorphic SI lacking the pollen-stigma dimorphism.
Lines 135-137. This comparison is meaningless as there is not phylogenetic information used here. Instead, it would be more valuable to explore the distribution of existence of different breeding systems in different morphs of the same species (if demonstrated, see above).
Lines 142-145. Why dimorphic populations were not explored? if they do not exist, that is intriguing: it is by chance that only one morph is forming each population? any selective values of morphs? I doubt it, but it should be explored. Probably they are different lineages, yet reproductively non-isolated, forming largely clonal populations, which offer a more parsimonious explanation. Additionally, 10 stems (genets, ramets? this is critical issue) is a very small sample to characterize a population in heterostylous species.
Lines 169-179. The numbers of samples in this paragraph (which are large indeed) does not refers to how many individual plants (genets) and this is critical.
Lines 189-191. I do not see what the rationale is to mix pollen from S- and L-plants to
do experimental intermorph pollinations. I assume that results are clearer with appropriate pollen donors of only the opposite morph. Why simulating random crosses? in fact you did not simulate that.
Lines 228 and thereafter (“Statistical analyses”). This section should include also details on analyses of floral morphology and any other data, in addition to those details on pollen tubes already included.
Lines 341-343. What is the meaning of this advantage if populations are composed a of a single morph? This is why is so important to study also dimorphic populations, if they exist.
Lines 353-354. Narcissus tazetta and N. papyraeus are not heterostylous, but style dimorphic, and their SI is not heteromorphic, but similar to gametophytic homomorphic SI.
Line 357-358. Ipomopsis aggregata is no homomorphic, but monomorphic. The difference is critical in heterostyly literature: monomorphism refers to one single morph in the population/species, whatever the herkogamy is; homomorphism refers to a monomorphic condition with no herkogamy.
Line 360: You should also mention that Raven (1979) explicitly mentioned that no Onagraceae is reported to have heterostyly.
Line 388 and thereafter. What is the significance of the advantage of cross-pollen tubes over self-pollen tubes if this type of cross does not occur in wild populations? I agree that this mechanism has been previously and frequently reported, but in monomorphic species.
Line 400. The concept of “in situ populations” is odd: I would suggest “wild populations”
Line 404-405. How do you discard that the small number of seeds produced by L-morph wild populations are not due to the pollen-transfer from nearby, unsampled S-morph populations as a result of natural legitimate cross-pollination between morphs?
Line 408 and thereafter. The number of seeds per se does not tell almost anything about sexual regeneration of populations, you need demographic data (number of seedlings, juveniles, adults) to address this important question of invasion ecology. Also, the balance between sexual and vegetative reproduction is crucial here.
Lines 419-426. What you mention, two breeding systems in the same species, is not mixed mating (see my comment above about appropriate terms). You need appropriate markers to demonstrate mixed mating (i.e., the relative number of seed sired by outcrossing and selfing in wild conditions).
Reference Gibbs PE 2014a and 2014b are the same
Reference Gibbs PE, Byran GW 1986 is unrelated with the topic. The first author is not the same as in the former ref.
Reference Takayama S, Isogai A 2005. Please change title to lower-case
Figure 1. Why are stamen measurements not provided? they are critical for definition of heterostyly as reciprocal herkogamy
Figure 6. This king of information is better provided as a table or bar diagram figure
Figure S1. If each point in the curve along time is derived from sampling, as I suppose, you should report error bars.
Figure S2. I do not see its value for understanding the manuscript.
On the more positive side, I must acknowledge the value of the study for plant biologists and botanists as the histological work about SI and its site of recognition is superb. If the work is properly addressed in the future, within the heterostyly field if it is finally demonstrated, or in a different context of different lineages with different histories, it will be a significant contribution.
Reviewed by anonymous reviewer 1, 27 Aug 2021
I consider the topic of this work adequate and interesting to be publised, however I think that some ideas must be reviewed:
A) Introduction:
· L72-L74: The authors expose: “Commonly, species with style polymorphism have a sporophytic heteromorphic (i.e. di- or tri-allelic) incompatibility system that prevents self-fertilization and crosses between individuals of the same floral morph (Barrett 2019).” However, there are several genera, as Lithodora, Glandora, Narcissus, in which stylar polymorphism (or heterostyly is not associated with the presence of an heteromorphic incompatibility system.
· L81-82: “It concerns both homomorphic and heterostylous species (Gibbs 2014a; Simon-Porcar et al. 2015).” The study of Simon-Porcar et al 2015 is focused on a stylar dimorphic species (Narcissus papyraceus); therefore, the term heterostylous in L81-82 should be replaced to “stylar polymorphic”.
· L85-87: “In the ovarian LSI heterostylous Narcissus spp. from the Amarilidacea, from 4 to 30% of their seed-sets result from self-fertilization (Barrett et al. 2004; Medrano et al. 2012; Simon-86 Porcar et al. 2015)” In the genus Narcissus there are 2 species which presents heterostyly and 12 or 13 that exhibit stylar-dimorphism. The data and the references that give us the author are from a stylar-dimorphic species (Simón-Porcar et al 2015) and a monomorphic species (Medrano et al 2012). Therefore, the term “heterostylous” in line 85-87 should be changed to stylar-polymorphism and the reference Medrano et al 2012, should be eliminated because that work is focused in Narcissus longispathus, which is a monomorphic species.
· L103-L108: The authors expose the floral architecture of both morph of this species, however they do not reveal the position of the stigma in bot morph; at this point I suspect that the short-styled morph exhibit the stigma below the two levels of anthers but I’m not sure about the long-styled morph, the stigma protrudes the two levels or anther or is it between the two levels or anthers?. Furthermore, it is not the only heterostylous species that exhibit to levels of anthers.
· L111: the authors exposed that 75% of the invasive populations worldwide are composed of L-plants. Could be this fact a consequence of vegetative reproduction? They do not say nothing about the frequency (or relevance) of vegetative vs sexual reproducition in invasive populations.
Materials and methods:
· L173-174: A brief description of the pollen morphology of this species should be given to understand why the authors decided to measure the diameter of the pollen grains.
· L174-L177: It is necessary to include a brief description of the principal differences between the stigmatic papillae in heterostylous species; and the authors should include how they are going to measure these differences.
· L197: I would like to know Why the authors fixed the flowers 2, 3, 7, 16 and 24 hours after the cross- or self-pollination. Are they following a specific protocol?
· L216: “We also assessed the rate of self-fertilisation in self-incompatible L-morph from in situ populations at the beginning of October.” L-morph should be replaced by L- and S-morph; Because They estimated the rate of self-fertilisation in both morphs.
· L216-L225: They collect seed from natural populations to estimate the rate of self-fertilization, however they do not specify if these flowers were caged or not and if they were not caged, how it is possible to be sure that crosses between different plant of the same morph were avoided?
· L232: It is not crucial but, in a GEE or GMM model, the authors could include random variables as the plant or the population.
Results:
· L250-254: The authors found differences in style length and width between both morphs, however in the Materials and Methods section they do not specify how they analyzed this observation and what is the goal.
Discussion:
· L341-L43: The authors exposed: “Yet, in the two Lgh floral morphs, inter-morph pollen tubes always elongated faster than self-pollen tubes, which may give advantage to intermorph crosses when inter-morph pollen is available”. This fact may have an effect on the stability of both morphs, specially for the L-morph in natural populations, and I think that I must be discussed.
· L353- L354, Narcissus tazetta and N. papyraceus are not heterostylous species. They exhibit stigma height dimorphism. In the genus Narcissus there are two heterostylous species: N. triandrius and N. albimarginatus. therefore, these species should be cited as stylar-dimorphic species or stylar-polymorphic species (if the authors include N. triandrus).
· L376-L385: I´m not totally agree with this observation and I´m not sure that this study prove that the residual number of seed that produces the L-morph after self-pollination can be a mechanism to maintain this morph in a natural population.
· L387-L309: The authors might give an explanation about why this fact is only observed in the S-morph and what is the role of this characteristic in the maintenance of both morphs in natural populations.
· As I told before, in the introduction section, it is important to include also in the discussion section a paragraph that compares rates vegetative and sexual reproduction in the invaders and native areas of this specie to understand the relevance of the mechanism described in this work.
· Finally, it could be interesting add a final paragraph in the discussion section to talk about the relevance of this work to control the invasion of this species.
https://doi.org/10.24072/pci.ecology.100330.rev12









