Direct submissions to PCI Ecology from bioRxiv.org are possible using the B2J service
Latest recommendations

| Id | Title * | Authors * | Abstract * | Picture * | Thematic fields * | Recommender | Reviewers▲ | Submission date | |
|---|---|---|---|---|---|---|---|---|---|
11 Mar 2021
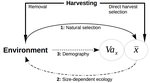
Size-dependent eco-evolutionary feedbacks in fisheriesEric Edeline and Nicolas Loeuille https://doi.org/10.1101/2020.04.03.022905“Hidden” natural selection and the evolution of body size in harvested stocksRecommended by Simon Blanchet based on reviews by Jean-François Arnoldi and 1 anonymous reviewerHumans are exploiting biological resources since thousands of years. Exploitation of biological resources has become particularly intense since the beginning of the 20th century and the steep increase in the worldwide human population size. Marine and freshwater fishes are not exception to that rule, and they have been (and continue to be) strongly harvested as a source of proteins for humans. For some species, fishery has been so intense that natural stocks have virtually collapsed in only a few decades. The worst example begin that of the Northwest Atlantic cod that has declined by more than 95% of its historical biomasses in only 20-30 years of intensive exploitation (Frank et al. 2005). These rapid and steep changes in biomasses have huge impacts on the entire ecosystems since species targeted by fisheries are often at the top of trophic chains (Frank et al. 2005). Beyond demographic impacts, fisheries also have evolutionary impacts on populations, which can also indirectly alter ecosystems (Uusi-Heikkilä et al. 2015; Palkovacs et al. 2018). Fishermen generally focus on the largest specimens, and hence exert a strong selective pressure against these largest fish (which is called “harvest selection”). There is now ample evidence that harvest selection can lead to rapid evolutionary changes in natural populations toward small individuals (Kuparinen & Festa-Bianchet 2017). These evolutionary changes are of course undesirable from a human perspective, and have attracted many scientific questions. Nonetheless, the consequence of harvest selection is not always observable in natural populations, and there are cases in which no phenotypic change (or on the contrary an increase in mean body size) has been observed after intense harvest pressures. In a conceptual Essay, Edeline and Loeuille (Edeline & Loeuille 2020) propose novel ideas to explain why the evolutionary consequences of harvest selection can be so diverse, and how a cross talk between ecological and evolutionary dynamics can explain patterns observed in natural stocks. The general and novel concept proposed by Edeline and Loeuille is actually as old as Darwin’s book; The Origin of Species (Darwin 1859). It is based on the simple idea that natural selection acting on harvested populations can actually be strong, and counter-balance (or on the contrary reinforce) the evolutionary consequence of harvest selection. Although simple, the idea that natural and harvest selection are jointly shaping contemporary evolution of exploited populations lead to various and sometimes complex scenarios that can (i) explain unresolved empirical patterns and (ii) refine predictions regarding the long-term viability of exploited populations. The Edeline and Loeuille’s crafty inspiration is that natural selection acting on exploited populations is itself an indirect consequence of harvest (Edeline & Loeuille 2020). They suggest that, by modifying the size structure of populations (a key parameter for ecological interactions), harvest indirectly alters interactions between populations and their biotic environment through competition and predation, which changes the ecological theatre and hence the selective pressures acting back to populations. They named this process “size-dependent eco-evolutionary feedback loops” and develop several scenarios in which these feedback loops ultimately deviate the evolutionary outcome of harvest selection from expectation. The scenarios they explore are based on strong theoretical knowledge, and range from simple ones in which a single species (the harvest species) is evolving to more complex (and realistic) ones in which multiple (e.g. the harvest species and its prey) species are co-evolving. I will not come into the details of each scenario here, and I will let the readers (re-)discovering the complex beauty of biological life and natural selection. Nonetheless, I will emphasize the importance of considering these eco-evolutionary processes altogether to fully grasp the response of exploited populations. Edeline and Loeuille convincingly demonstrate that reduced body size due to harvest selection is obviously not the only response of exploited fish populations when natural selection is jointly considered (Edeline & Loeuille 2020). On the contrary, they show that –under some realistic ecological circumstances relaxing exploitative competition due to reduced population densities- natural selection can act antagonistically, and hence favour stable body size in exploited populations. Although this seems further desirable from a human perspective than a downsizing of exploited populations, it is actually mere window dressing as Edeline and Loeuille further showed that this response is accompanied by an erosion of the evolvability –and hence a lowest probability of long-term persistence- of these exploited populations. Humans, by exploiting biological resources, are breaking the relative equilibrium of complex entities, and the response of populations to this disturbance is itself often complex and heterogeneous. In this Essay, Edeline and Loeuille provide –under simple terms- the theoretical and conceptual bases required to improve predictions regarding the evolutionary responses of natural populations to exploitation by humans (Edeline & Loeuille 2020). An important next step will be to generate data and methods allowing confronting the empirical reality to these novel concepts (e.g. (Monk et al. 2021), so as to identify the most likely evolutionary scenarios sustaining biological responses of exploited populations, and hence to set the best management plans for the long-term sustainability of these populations. References Darwin, C. (1859). On the Origin of Species by Means of Natural Selection. John Murray, London. Edeline, E. & Loeuille, N. (2021) Size-dependent eco-evolutionary feedbacks in fisheries. bioRxiv, 2020.04.03.022905, ver. 4 peer-reviewed and recommended by PCI Ecology. doi: https://doi.org/10.1101/2020.04.03.022905 Frank, K.T., Petrie, B., Choi, J. S. & Leggett, W.C. (2005). Trophic Cascades in a Formerly Cod-Dominated Ecosystem. Science, 308, 1621–1623. doi: https://doi.org/10.1126/science.1113075 Kuparinen, A. & Festa-Bianchet, M. (2017). Harvest-induced evolution: insights from aquatic and terrestrial systems. Philos. Trans. R. Soc. B Biol. Sci., 372, 20160036. doi: https://doi.org/10.1098/rstb.2016.0036 Monk, C.T., Bekkevold, D., Klefoth, T., Pagel, T., Palmer, M. & Arlinghaus, R. (2021). The battle between harvest and natural selection creates small and shy fish. Proc. Natl. Acad. Sci., 118, e2009451118. doi: https://doi.org/10.1073/pnas.2009451118 Palkovacs, E.P., Moritsch, M.M., Contolini, G.M. & Pelletier, F. (2018). Ecology of harvest-driven trait changes and implications for ecosystem management. Front. Ecol. Environ., 16, 20–28. doi: https://doi.org/10.1002/fee.1743 Uusi-Heikkilä, S., Whiteley, A.R., Kuparinen, A., Matsumura, S., Venturelli, P.A., Wolter, C., et al. (2015). The evolutionary legacy of size-selective harvesting extends from genes to populations. Evol. Appl., 8, 597–620. doi: https://doi.org/10.1111/eva.12268 | Size-dependent eco-evolutionary feedbacks in fisheries | Eric Edeline and Nicolas Loeuille | <p>Harvesting may drive body downsizing along with population declines and decreased harvesting yields. These changes are commonly construed as direct consequences of harvest selection, where small-bodied, early-reproducing individuals are immedia... |  | Biodiversity, Community ecology, Competition, Eco-evolutionary dynamics, Evolutionary ecology, Food webs, Interaction networks, Life history, Population ecology, Theoretical ecology | Simon Blanchet | 2020-04-03 16:14:05 | View | |
12 Oct 2020

Insect herbivory on urban trees: Complementary effects of tree neighbours and predationAlex Stemmelen, Alain Paquette, Marie-Lise Benot, Yasmine Kadiri, Hervé Jactel, Bastien Castagneyrol https://doi.org/10.1101/2020.04.15.042317Tree diversity is associated with reduced herbivory in urban forestRecommended by Ruth Arabelle Hufbauer and Ian Pearse based on reviews by Ian Pearse and Freerk MollemanUrban ecology, the study of ecological systems in our increasingly urbanized world, is crucial to planning and redesigning cities to enhance ecosystem services (Kremer et al. 2016), human health and well-being and further conservation goals (Dallimer et al. 2012). Urban trees are a crucial component of urban streets and parks that provide shade and cooling through evapotranspiration (Fung and Jim 2019), improve air quality (Lai and Kontokosta 2019), help control storm water (Johnson and Handel 2016), and conserve wildlife (Herrmann et al. 2012; de Andrade et al. 2020). References Airola, D. and Greco, S. (2019). Birds and oaks in California’s urban forest. Int. Oaks, 30, 109–116. | Insect herbivory on urban trees: Complementary effects of tree neighbours and predation | Alex Stemmelen, Alain Paquette, Marie-Lise Benot, Yasmine Kadiri, Hervé Jactel, Bastien Castagneyrol | <p>Insect herbivory is an important component of forest ecosystems functioning and can affect tree growth and survival. Tree diversity is known to influence insect herbivory in natural forest, with most studies reporting a decrease in herbivory wi... |  | Biodiversity, Biological control, Community ecology, Ecosystem functioning, Herbivory | Ruth Arabelle Hufbauer | 2020-04-20 13:49:36 | View | |
30 Sep 2020
How citizen science could improve Species Distribution Models and their independent assessmentFlorence Matutini, Jacques Baudry, Guillaume Pain, Morgane Sineau, Josephine Pithon https://doi.org/10.1101/2020.06.02.129536Citizen science contributes to SDM validationRecommended by Francisco Lloret based on reviews by Maria Angeles Perez-Navarro and 1 anonymous reviewerCitizen science is becoming an important piece for the acquisition of scientific knowledge in the fields of natural sciences, and particularly in the inventory and monitoring of biodiversity (McKinley et al. 2017). The information generated with the collaboration of citizens has an evident importance in conservation, by providing information on the state of populations and habitats, helping in mitigation and restoration actions, and very importantly contributing to involve society in conservation (Brown and Williams 2019).
An obvious advantage of these initiatives is the ability to mobilize human resources on a large territorial scale and in the medium term, which would otherwise be difficult to finance. The resulting increasing information then can be processed with advanced computational techniques (Hochachka et al 2012; Kelling et al. 2015), thus improving our interpretation of the distribution of species. Specifically, the ability to obtain information on a large territorial scale can be integrated into studies based on Species Distribution Models SDMs. One of the common problems with SDMs is that they often work from species occurrences that have been opportunistically recorded, either by professionals or amateurs. A great challenge for data obtained from non-professional citizens, however, remains to ensure its standardization and quality (Kosmala et al. 2016). This requires a clear and effective design, solid volunteer training, and a high level of coordination that turns out to be complex (Brown and Williams 2019). Finally, it is essential to perform a quality validation following scientifically recognized standards, since they are often conditioned by errors and biases in obtaining information (Bird et al. 2014). There are two basic approaches to obtain the necessary data for this validation: getting it from an external source (external validation), or allocating a part of the database itself (internal validation or cross-validation) to this function. References [1] Bird TJ et al. (2014) Statistical solutions for error and bias in global citizen science datasets. Biological Conservation 173: 144-154. doi: 10.1016/j.biocon.2013.07.037 | How citizen science could improve Species Distribution Models and their independent assessment | Florence Matutini, Jacques Baudry, Guillaume Pain, Morgane Sineau, Josephine Pithon | <p>Species distribution models (SDM) have been increasingly developed in recent years but their validity is questioned. Their assessment can be improved by the use of independent data but this can be difficult to obtain and prohibitive to collect.... | Biodiversity, Biogeography, Conservation biology, Habitat selection, Spatial ecology, Metacommunities & Metapopulations, Species distributions, Statistical ecology | Francisco Lloret | 2020-06-03 09:36:34 | View | ||
28 Sep 2020
The dynamics of spawning acts by a semelparous fish and its associated energetic costsCédric Tentelier, Colin Bouchard, Anaïs Bernardin, Amandine Tauzin, Jean-Christophe Aymes, Frédéric Lange, Charlotte Recapet, Jacques Rives https://doi.org/10.1101/436295Extreme weight loss: when accelerometer could reveal reproductive investment in a semelparous fish speciesRecommended by Francois-Xavier Dechaume-Moncharmont based on reviews by Aidan Jonathan Mark Hewison, Loïc Teulier and 1 anonymous reviewer based on reviews by Aidan Jonathan Mark Hewison, Loïc Teulier and 1 anonymous reviewer
Continuous observation of animal behaviour could be quite a challenge in the field, and the situation becomes even more complicated with aquatic species mostly active at night. In such cases, biologging techniques are real game changers in ecology, behavioural ecology or eco-physiology. An accelerating number of methodological applications of these tools in natural condition are thus published each year [1]. Biologging is not limited to movement ecology. For instance, fine grain information about energy expenditure can be inferred from body acceleration [2], and accelerometers has already proven useful in monitoring reproductive costs in some fish species [3,4]. The first part of the study by Tentelier et al. [5] is in line with this growing literature. It describes measurements of energy expenditure during reproduction in a fish species, Allis shad (Alosa Alosa), based on tail beat frequency and occurrence of spawning acts. The study has been convincingly conducted, and the results are important for fish biologists. But this is not the whole story: the authors added to this otherwise classical study a very original and insightful analysis which deserves closer interest. References [1] Börger L, Bijleveld AI, Fayet AL, Machovsky‐Capuska GE, Patrick SC, Street GM and Vander Wal E. (2020) Biologging special feature. J. Anim. Ecol. 89, 6–15. 10.1111/1365-2656.13163 | The dynamics of spawning acts by a semelparous fish and its associated energetic costs | Cédric Tentelier, Colin Bouchard, Anaïs Bernardin, Amandine Tauzin, Jean-Christophe Aymes, Frédéric Lange, Charlotte Recapet, Jacques Rives | <p>1. During the reproductive season, animals have to manage both their energetic budget and gamete stock. In particular, for semelparous capital breeders with determinate fecundity and no parental care other than gametic investment, the depletion... | Behaviour & Ethology, Freshwater ecology, Life history | Francois-Xavier Dechaume-Moncharmont | 2020-06-04 15:18:56 | View | ||
21 Dec 2020

Influence of local landscape and time of year on bat-road collision risksCharlotte Roemer, Aurélie Coulon, Thierry Disca, and Yves Bas https://doi.org/10.1101/2020.07.15.204115Assessing bat-vehicle collision risks using acoustic 3D trackingRecommended by Gloriana Chaverri based on reviews by Mark Brigham and ? based on reviews by Mark Brigham and ?
The loss of biodiversity is an issue of great concern, especially if the extinction of species or the loss of a large number of individuals within populations results in a loss of critical ecosystem services. We know that the most important threat to most species is habitat loss and degradation (Keil et al., 2015; Pimm et al., 2014); the latter can be caused by multiple anthropogenic activities, including pollution, introduction of invasive species and fragmentation (Brook et al., 2008; Scanes, 2018). Roads are a major cause of habitat fragmentation, isolating previously connected populations and being a direct source of mortality for animals that attempt to cross them (Spellberg, 1998). References [1] Bartonička T, Andrášik R, Duľa M, Sedoník J, Bíl M (2018) Identification of local factors causing clustering of animal-vehicle collisions. The Journal of Wildlife Management, 82, 940–947. https://doi.org/10.1002/jwmg.21467 | Influence of local landscape and time of year on bat-road collision risks | Charlotte Roemer, Aurélie Coulon, Thierry Disca, and Yves Bas | <p>Roads impact bat populations through habitat loss and collisions. High quality habitats particularly increase bat mortalities on roads, yet many questions remain concerning how local landscape features may influence bat behaviour and lead to hi... |  | Behaviour & Ethology, Biodiversity, Conservation biology, Human impact, Landscape ecology | Gloriana Chaverri | 2020-07-20 10:56:29 | View | |
18 Dec 2020

Once upon a time in the far south: Influence of local drivers and functional traits on plant invasion in the harsh sub-Antarctic islandsManuele Bazzichetto, François Massol, Marta Carboni, Jonathan Lenoir, Jonas Johan Lembrechts, Rémi Joly, David Renault https://doi.org/10.1101/2020.07.19.210880A meaningful application of species distribution models and functional traits to understand invasion dynamicsRecommended by Joaquín Hortal based on reviews by Paula Matos and Peter Convey based on reviews by Paula Matos and Peter Convey
Polar and subpolar regions are fragile environments, where the introduction of alien species may completely change ecosystem dynamics if the alien species become keystone species (e.g. Croll, 2005). The increasing number of human visits, together with climate change, are favouring the introduction and settling of new invaders to these regions, particularly in Antarctica (Hughes et al. 2015). Within this context, the joint use of Species Distribution Models (SDM) –to assess the areas potentially suitable for the aliens– with other measures of the potential to become successful invaders can inform on the need for devoting specific efforts to eradicate these new species before they become naturalized (e.g. Pertierra et al. 2016). References Austin, M. P., Nicholls, A. O., and Margules, C. R. (1990). Measurement of the realized qualitative niche: environmental niches of five Eucalyptus species. Ecological Monographs, 60(2), 161-177. doi: https://doi.org/10.2307/1943043 | Once upon a time in the far south: Influence of local drivers and functional traits on plant invasion in the harsh sub-Antarctic islands | Manuele Bazzichetto, François Massol, Marta Carboni, Jonathan Lenoir, Jonas Johan Lembrechts, Rémi Joly, David Renault | <p>Aim Here, we aim to: (i) investigate the local effect of environmental and human-related factors on alien plant invasion in sub-Antarctic islands; (ii) explore the relationship between alien species features and their dependence on anthropogeni... |  | Biogeography, Biological invasions, Spatial ecology, Metacommunities & Metapopulations, Species distributions | Joaquín Hortal | 2020-07-21 21:13:08 | View | |
26 Apr 2021
Experimental test for local adaptation of the rosy apple aphid (Dysaphis plantaginea) during its recent rapid colonization on its cultivated apple host (Malus domestica) in EuropeOlvera-Vazquez S.G., Alhmedi A., Miñarro M., Shykoff J. A., Marchadier E., Rousselet A., Remoué C., Gardet R., Degrave A. , Robert P. , Chen X., Porcher J., Giraud T., Vander-Mijnsbrugge K., Raffoux X., Falque M., Alins, G., Didelot F., Beliën T., Dapena E., Lemarquand A. and Cornille A. https://forgemia.inra.fr/amandine.cornille/local_adaptation_dpA planned experiment on local adaptation in a host-parasite system: is adaptation to the host linked to its recent domestication?Recommended by Eric Petit based on reviews by Sharon Zytynska, Alex Stemmelen and 1 anonymous reviewerLocal adaptation shall occur whenever selective pressures vary across space and overwhelm the effects of gene flow and local extinctions (Kawecki and Ebert 2004). Because the intimate interaction that characterizes their relationship exerts a strong selective pressure on both partners, host-parasite systems represent a classical example in which local adaptation is expected from rapidly evolving parasites adapting to more evolutionary constrained hosts (Kaltz and Shykoff 1998). Such systems indeed represent a large proportion of the study-cases in local adaptation research (Runquist et al. 2020). Biotic interactions intervene in many environment-related societal challenges, so that understanding when and how local adaptation arises is important not only for understanding evolutionary dynamics but also for more applied questions such as the control of agricultural pests, biological invasions, or pathogens (Parker and Gilbert 2004). The exact conditions under which local adaptation does occur and can be detected is however still the focus of many theoretical, methodological and empirical studies (Blanquart et al. 2013, Hargreaves et al. 2020, Hoeksema and Forde 2008, Nuismer and Gandon 2008, Richardson et al. 2014). A recent review that evaluates investigations that examined the combined influence of biotic and abiotic factors on local adaptation reaches partial conclusions about their relative importance in different contexts and underlines the many traps that one has to avoid in such studies (Runquist et al. 2020). The authors of this review emphasize that one should evaluate local adaptation using wild-collected strains or populations and over multiple generations, on environmental gradients that span natural ranges of variation for both biotic and abiotic factors, in a theory-based hypothetico-deductive framework that helps interpret the outcome of experiments. These multiple targets are not easy to reach in each local adaptation experiment given the diversity of systems in which local adaptation may occur. Improving research practices may also help better understand when and where local adaptation does occur by adding controls over p-hacking, HARKing or publication bias, which is best achieved when hypotheses, date collection and analytical procedures are known before the research begins (Chambers et al. 2014). In this regard, the route taken by Olvera-Vazquez et al. (2021) is interesting. They propose to investigate whether the rosy aphid (Dysaphis plantaginea) recently adapted to its cultivated host, the apple tree (Malus domestica), and chose to pre-register their hypotheses and planned experiments on PCI Ecology (Peer Community In 2020). Though not fulfilling all criteria mentioned by Runquist et al. (2020), they clearly state five hypotheses that all relate to the local adaptation of this agricultural pest to an economically important fruit tree, and describe in details a powerful, randomized experiment, including how data will be collected and analyzed. The experimental set-up includes comparisons between three sites located along a temperature transect that also differ in local edaphic and biotic factors, and contrasts wild and domesticated apple trees that originate from the three sites and were both planted in the local, sympatric site, and transplanted to allopatric sites. Beyond enhancing our knowledge on local adaptation, this experiment will also test the general hypothesis that the rosy aphid recently adapted to Malus sp. after its domestication, a question that population genetic analyses was not able to answer (Olvera-Vazquez et al. 2020). References Blanquart F, Kaltz O, Nuismer SL, Gandon S (2013) A practical guide to measuring local adaptation. Ecology Letters, 16, 1195–1205. https://doi.org/10.1111/ele.12150 Briscoe Runquist RD, Gorton AJ, Yoder JB, Deacon NJ, Grossman JJ, Kothari S, Lyons MP, Sheth SN, Tiffin P, Moeller DA (2019) Context Dependence of Local Adaptation to Abiotic and Biotic Environments: A Quantitative and Qualitative Synthesis. The American Naturalist, 195, 412–431. https://doi.org/10.1086/707322 Chambers CD, Feredoes E, Muthukumaraswamy SD, Etchells PJ, Chambers CD, Feredoes E, Muthukumaraswamy SD, Etchells PJ (2014) Instead of “playing the game” it is time to change the rules: Registered Reports at <em>AIMS Neuroscience</em> and beyond. AIMS Neuroscience, 1, 4–17. https://doi.org/10.3934/Neuroscience.2014.1.4 Hargreaves AL, Germain RM, Bontrager M, Persi J, Angert AL (2019) Local Adaptation to Biotic Interactions: A Meta-analysis across Latitudes. The American Naturalist, 195, 395–411. https://doi.org/10.1086/707323 Hoeksema JD, Forde SE (2008) A Meta‐Analysis of Factors Affecting Local Adaptation between Interacting Species. The American Naturalist, 171, 275–290. https://doi.org/10.1086/527496 Kaltz O, Shykoff JA (1998) Local adaptation in host–parasite systems. Heredity, 81, 361–370. https://doi.org/10.1046/j.1365-2540.1998.00435.x Kawecki TJ, Ebert D (2004) Conceptual issues in local adaptation. Ecology Letters, 7, 1225–1241. https://doi.org/10.1111/j.1461-0248.2004.00684.x Nuismer SL, Gandon S (2008) Moving beyond Common‐Garden and Transplant Designs: Insight into the Causes of Local Adaptation in Species Interactions. The American Naturalist, 171, 658–668. https://doi.org/10.1086/587077 Olvera-Vazquez SG, Remoué C, Venon A, Rousselet A, Grandcolas O, Azrine M, Momont L, Galan M, Benoit L, David G, Alhmedi A, Beliën T, Alins G, Franck P, Haddioui A, Jacobsen SK, Andreev R, Simon S, Sigsgaard L, Guibert E, Tournant L, Gazel F, Mody K, Khachtib Y, Roman A, Ursu TM, Zakharov IA, Belcram H, Harry M, Roth M, Simon JC, Oram S, Ricard JM, Agnello A, Beers EH, Engelman J, Balti I, Salhi-Hannachi A, Zhang H, Tu H, Mottet C, Barrès B, Degrave A, Razmjou J, Giraud T, Falque M, Dapena E, Miñarro M, Jardillier L, Deschamps P, Jousselin E, Cornille A (2020) Large-scale geographic survey provides insights into the colonization history of a major aphid pest on its cultivated apple host in Europe, North America and North Africa. bioRxiv, 2020.12.11.421644. https://doi.org/10.1101/2020.12.11.421644 Olvera-Vazquez S.G., Alhmedi A., Miñarro M., Shykoff J. A., Marchadier E., Rousselet A., Remoué C., Gardet R., Degrave A. , Robert P. , Chen X., Porcher J., Giraud T., Vander-Mijnsbrugge K., Raffoux X., Falque M., Alins, G., Didelot F., Beliën T., Dapena E., Lemarquand A. and Cornille A. (2021) Experimental test for local adaptation of the rosy apple aphid (Dysaphis plantaginea) to its host (Malus domestica) and to its climate in Europe. In principle recommendation by Peer Community In Ecology. https://forgemia.inra.fr/amandine.cornille/local_adaptation_dp, ver. 4. Parker IM, Gilbert GS (2004) The Evolutionary Ecology of Novel Plant-Pathogen Interactions. Annual Review of Ecology, Evolution, and Systematics, 35, 675–700. https://doi.org/10.1146/annurev.ecolsys.34.011802.132339 Peer Community In. (2020, January 15). Submit your preregistration to Peer Community In for peer review. https://peercommunityin.org/2020/01/15/submit-your-preregistration-to-peer-community-in-for-peer-review/ Richardson JL, Urban MC, Bolnick DI, Skelly DK (2014) Microgeographic adaptation and the spatial scale of evolution. Trends in Ecology & Evolution, 29, 165–176. https://doi.org/10.1016/j.tree.2014.01.002 | Experimental test for local adaptation of the rosy apple aphid (Dysaphis plantaginea) during its recent rapid colonization on its cultivated apple host (Malus domestica) in Europe | Olvera-Vazquez S.G., Alhmedi A., Miñarro M., Shykoff J. A., Marchadier E., Rousselet A., Remoué C., Gardet R., Degrave A. , Robert P. , Chen X., Porcher J., Giraud T., Vander-Mijnsbrugge K., Raffoux X., Falque M., Alins, G., Didelot F., Beliën T.,... | <p style="text-align: justify;">Understanding the extent of local adaptation in natural populations and the mechanisms enabling populations to adapt to their environment is a major avenue in ecology research. Host-parasite interaction is widely se... | Evolutionary ecology, Preregistrations | Eric Petit | 2020-07-26 18:31:42 | View | ||
06 May 2021

Trophic niche of the invasive gregarious species Crepidula fornicata, in relation to ontogenic changesThibault Androuin, Stanislas F. Dubois, Cédric Hubas, Gwendoline Lefebvre, Fabienne Le Grand, Gauthier Schaal, Antoine Carlier https://doi.org/10.1101/2020.07.30.229021A lack of clear dietary differences between ontogenetic stages of invasive slippersnails provides important insights into resource use and potential inter- and intra-specific competitionRecommended by Matthew Bracken based on reviews by 2 anonymous reviewersThe slippersnail (Crepidula fornicata), originally from the eastern coast of North America, has invaded European coastlines from Norway to the Mediterranean Sea [1]. This species is capable of achieving incredibly high densities (up to several thousand individuals per square meter) and likely has major impacts on a variety of community- and ecosystem-level processes, including alteration of carbon and nitrogen fluxes and competition with native suspension feeders [2]. Given this potential for competition, it is important to understand the diet of C. fornicata and its potential overlap with native species. However, previous research on the diet of C. fornicata and related species suggests that the types of food consumed may change with age [3, 4]. This species has an unusual reproductive strategy. It is a sequential hermaphrodite, which begins life as a somewhat mobile male but eventually slows down to become sessile. Sessile individuals form stacks of up to 10 or more individuals, with larger individuals on the bottom of the stack, and decreasingly smaller individuals piled on top. Snails at the bottom of the stack are female, whereas snails at the top of the stack are male; when the females die, the largest males become female [5]. Thus, understanding these potential ontogenetic dietary shifts has implications for both intraspecific (juvenile vs. male vs. female) and interspecific competition associated with an abundant, invasive species. To this end, Androuin and colleagues evaluated the stable-isotope (d13C and d15N) and fatty-acid profiles of food sources and different life-history stages of C. fornicata [6]. Based on previous work highlighting the potential for life-history changes in the diet of this species [3,4], they hypothesized that C. fornicata would shift its diet as it aged and predicted that this shift would be reflected in changes in its stable-isotope and fatty-acid profiles. The authors found that potential food sources (biofilm, suspended particulate organic matter, and superficial sedimentary organic matter) differed substantially in both stable-isotope and fatty-acid signatures. However, whereas fatty-acid profiles changed substantially with age, there was no shift in the stable-isotope signatures. Because stable-isotope differences between food sources were not reflected in differences between life-history stages, the authors conservatively concluded that there was insufficient evidence for a diet shift with age. The ontogenetic shifts in fatty-acid profiles were intriguing, but the authors suggested that these reflected age-related physiological changes rather than changes in diet. The authors’ work highlights the need to consider potential changes in the roles of invasive species with age, especially when evaluating interactions with native species. In this case, C. fornicata consumed a variety of food sources, including both benthic and particulate organic matter, regardless of age. The carbon stable-isotope signature of C. fornicata overlaps with those of several native suspension- and deposit-feeding species in the region [7], suggesting the possibility of resource competition, especially given the high abundances of this invader. This contribution demonstrates the potential difficulty of characterizing the impacts of an abundant invasive species with a complex life-history strategy. Like many invasive species, C. fornicata appears to be a dietary generalist, which likely contributes to its success in establishing and thriving in a variety of locations [8].
References [1] Blanchard M (1997) Spread of the slipper limpet Crepidula fornicata (L. 1758) in Europe. Current state dans consequences. Scientia Marina, 61, 109–118. Open Access version : https://archimer.ifremer.fr/doc/00423/53398/54271.pdf [2] Martin S, Thouzeau G, Chauvaud L, Jean F, Guérin L, Clavier J (2006) Respiration, calcification, and excretion of the invasive slipper limpet, Crepidula fornicata L.: Implications for carbon, carbonate, and nitrogen fluxes in affected areas. Limnology and Oceanography, 51, 1996–2007. https://doi.org/10.4319/lo.2006.51.5.1996 [3] Navarro JM, Chaparro OR (2002) Grazing–filtration as feeding mechanisms in motile specimens of Crepidula fecunda (Gastropoda: Calyptraeidae). Journal of Experimental Marine Biology and Ecology, 270, 111–122. https://doi.org/10.1016/S0022-0981(02)00013-8 [4] Yee AK, Padilla DK (2015) Allometric Scaling of the Radula in the Atlantic Slippersnail Crepidula fornicata. Journal of Shellfish Research, 34, 903–907. https://doi.org/10.2983/035.034.0320 [5] Collin R (1995) Sex, Size, and Position: A Test of Models Predicting Size at Sex Change in the Protandrous Gastropod Crepidula fornicata. The American Naturalist, 146, 815–831. https://doi.org/10.1086/285826 [6] Androuin T, Dubois SF, Hubas C, Lefebvre G, Grand FL, Schaal G, Carlier A (2021) Trophic niche of the invasive gregarious species Crepidula fornicata, in relation to ontogenic changes. bioRxiv, 2020.07.30.229021, ver. 4 peer-reviewed and recommended by Peer Community in Ecology. https://doi.org/10.1101/2020.07.30.229021 [7] Dauby P, Khomsi A, Bouquegneau J-M (1998) Trophic Relationships within Intertidal Communities of the Brittany Coasts: A Stable Carbon Isotope Analysis. Journal of Coastal Research, 14, 1202–1212. Retrieved May 4, 2021, from http://www.jstor.org/stable/4298880 [8] Machovsky-Capuska GE, Senior AM, Simpson SJ, Raubenheimer D (2016) The Multidimensional Nutritional Niche. Trends in Ecology & Evolution, 31, 355–365. https://doi.org/10.1016/j.tree.2016.02.009
| Trophic niche of the invasive gregarious species Crepidula fornicata, in relation to ontogenic changes | Thibault Androuin, Stanislas F. Dubois, Cédric Hubas, Gwendoline Lefebvre, Fabienne Le Grand, Gauthier Schaal, Antoine Carlier | <p style="text-align: justify;">The slipper limpet Crepidula fornicata is a common and widespread invasive gregarious species along the European coast. Among its life-history traits, well-documented ontogenic changes in behavior (i.e., motile male... |  | Food webs, Life history, Marine ecology | Matthew Bracken | 2020-08-01 23:55:57 | View | |
22 Mar 2021

Host-mediated, cross-generational intraspecific competition in a herbivore speciesBastien Castagneyrol, Inge van Halder, Yasmine Kadiri, Laura Schillé, Hervé Jactel https://doi.org/10.1101/2020.07.30.228544Plants preserve the ghost of competition past for herbivores, but mothers don’t careRecommended by Sara Magalhães based on reviews by Inês Fragata and Raul Costa-PereiraSome biological hypotheses are widely popular, so much so that we tend to forget their original lack of success. This is particularly true for hypotheses with catchy names. The ‘Ghost of competition past’ is part of the title of a paper by the great ecologist, JH Connell, one of the many losses of 2020 (Connell 1980). The hypothesis states that, even though we may not detect competition in current populations, their traits and distributions may be shaped by past competition events. Although this hypothesis has known a great success in the ecological literature, the original paper actually ends with “I will no longer be persuaded by such invoking of "the Ghost of Competition Past"”. Similarly, the hypothesis that mothers of herbivores choose host plants where their offspring will have a higher fitness was proposed by John Jaenike in 1978 (Jaenike 1978), and later coined the ‘mother knows best’ hypothesis. The hypothesis was readily questioned or dismissed: “Mother doesn't know best” (Courtney and Kibota 1990), or “Does mother know best?” (Valladares and Lawton 1991), but remains widely popular. It thus seems that catchy names (and the intuitive ideas behind them) have a heuristic value that is independent from the original persuasion in these ideas and the accumulation of evidence that followed it. The paper by Castagneryol et al. (2021) analyses the preference-performance relationship in the box tree moth (BTM) Cydalima perspectalis, after defoliation of their host plant, the box tree, by conspecifics. It thus has bearings on the two previously mentioned hypotheses. Specifically, they created an artificial population of potted box trees in a greenhouse, in which 60 trees were infested with BTM third instar larvae, whereas 61 were left uninfested. One week later, these larvae were removed and another three weeks later, they released adult BTM females and recorded their host choice by counting egg clutches laid by these females on the plants. Finally, they evaluated the effect of previously infested vs uninfested plants on BTM performance by measuring the weight of third instar larvae that had emerged from those eggs. This experimental design was adopted because BTM is a multivoltine species. When the second generation of BTM arrives, plants have been defoliated by the first generation and did not fully recover. Indeed, Castagneryol et al. (2021) found that larvae that developed on previously infested plants were much smaller than those developing on uninfested plants, and the same was true for the chrysalis that emerged from those larvae. This provides unequivocal evidence for the existence of a ghost of competition past in this system. However, the existence of this ghost still does not result in a change in the distribution of BTM, precisely because mothers do not know best: they lay as many eggs on plants previously infested than on uninfested plants. The demonstration that the previous presence of a competitor affects the performance of this herbivore species confirms that ghosts exist. However, whether this entails that previous (interspecific) competition shapes species distributions, as originally meant, remains an open question. Species phenology may play an important role in exposing organisms to the ghost, as this time-lagged competition may have been often overlooked. It is also relevant to try to understand why mothers don’t care in this, and other systems. One possibility is that they will have few opportunities to effectively choose in the real world, due to limited dispersal or to all plants being previously infested. References Castagneyrol, B., Halder, I. van, Kadiri, Y., Schillé, L. and Jactel, H. (2021) Host-mediated, cross-generational intraspecific competition in a herbivore species. bioRxiv, 2020.07.30.228544, ver. 5 peer-reviewed and recommended by PCI Ecology. doi: https://doi.org/10.1101/2020.07.30.228544 Connell, J. H. (1980). Diversity and the coevolution of competitors, or the ghost of competition past. Oikos, 131-138. doi: https://doi.org/10.2307/3544421 Courtney, S. P. and Kibota, T. T. (1990) in Insect-plant interactions (ed. Bernays, E.A.) 285-330. Jaenike, J. (1978). On optimal oviposition behavior in phytophagous insects. Theoretical population biology, 14(3), 350-356. doi: https://doi.org/10.1016/0040-5809(78)90012-6 Valladares, G., and Lawton, J. H. (1991). Host-plant selection in the holly leaf-miner: does mother know best?. The Journal of Animal Ecology, 227-240. doi: https://doi.org/10.2307/5456
| Host-mediated, cross-generational intraspecific competition in a herbivore species | Bastien Castagneyrol, Inge van Halder, Yasmine Kadiri, Laura Schillé, Hervé Jactel | <p>Conspecific insect herbivores co-occurring on the same host plant interact both directly through interference competition and indirectly through exploitative competition, plant-mediated interactions and enemy-mediated interactions. However, the... |  | Competition, Herbivory, Zoology | Sara Magalhães | 2020-08-03 15:50:23 | View | |
14 Jan 2021
Consistent variations in personality traits and their potential for genetic improvement of biocontrol agents: Trichogramma evanescens as a case studySilène Lartigue, Myriam Yalaoui, Jean Belliard, Claire Caravel, Louise Jeandroz, Géraldine Groussier, Vincent Calcagno, Philippe Louâpre, François-Xavier Dechaume-Moncharmont, Thibaut Malausa and Jérôme Moreau https://doi.org/10.1101/2020.08.21.257881Tell us how you can be, and we’ll make you better: exploiting genetic variability in personality traits to improve top-down control of agricultural pestsRecommended by Marta Montserrat based on reviews by Bart A Pannebakker, François Dumont, Joshua Patrick Byrne and Ana Pimenta Goncalves PereiraAgriculture in the XXI century faces the huge challenge of having to provide food to a rapidly growing human population, which is expected to reach 10.9 billion in 2100 (UUNN 2019), by means of practices and methods that guarantee crop sustainability, human health safety, and respect to the environment (UUNN 2015). Such regulation by the United Nations ultimately entails that agricultural scientists are urged to design strategies and methods that effectively minimize the use of harmful chemical products to control pest populations and to improve soil quality. References Bielza, P., Balanza, V., Cifuentes, D. and Mendoza, J. E. (2020). Challenges facing arthropod biological control: Identifying traits for genetic improvement of predators in protected crops. Pest Manag Sci. doi: https://doi.org/10.1002/ps.5857 | Consistent variations in personality traits and their potential for genetic improvement of biocontrol agents: Trichogramma evanescens as a case study | Silène Lartigue, Myriam Yalaoui, Jean Belliard, Claire Caravel, Louise Jeandroz, Géraldine Groussier, Vincent Calcagno, Philippe Louâpre, François-Xavier Dechaume-Moncharmont, Thibaut Malausa and Jérôme Moreau | <p>Improvements in the biological control of agricultural pests require improvements in the phenotyping methods used by practitioners to select efficient biological control agent (BCA) populations in industrial rearing or field conditions. Consist... | Agroecology, Behaviour & Ethology, Biological control, Evolutionary ecology, Life history | Marta Montserrat | 2020-08-24 10:40:03 | View |
MANAGING BOARD
Julia Astegiano
Tim Coulson
Anna Eklof
Dominique Gravel
François Massol
Ben Phillips
Cyrille Violle










