Direct submissions to PCI Ecology from bioRxiv.org are possible using the B2J service
Latest recommendations

| Id | Title * | Authors * | Abstract * | Picture * | Thematic fields * | Recommender▲ | Reviewers | Submission date | |
|---|---|---|---|---|---|---|---|---|---|
12 Oct 2020

Insect herbivory on urban trees: Complementary effects of tree neighbours and predationAlex Stemmelen, Alain Paquette, Marie-Lise Benot, Yasmine Kadiri, Hervé Jactel, Bastien Castagneyrol https://doi.org/10.1101/2020.04.15.042317Tree diversity is associated with reduced herbivory in urban forestRecommended by Ruth Arabelle Hufbauer and Ian Pearse based on reviews by Ian Pearse and Freerk MollemanUrban ecology, the study of ecological systems in our increasingly urbanized world, is crucial to planning and redesigning cities to enhance ecosystem services (Kremer et al. 2016), human health and well-being and further conservation goals (Dallimer et al. 2012). Urban trees are a crucial component of urban streets and parks that provide shade and cooling through evapotranspiration (Fung and Jim 2019), improve air quality (Lai and Kontokosta 2019), help control storm water (Johnson and Handel 2016), and conserve wildlife (Herrmann et al. 2012; de Andrade et al. 2020). References Airola, D. and Greco, S. (2019). Birds and oaks in California’s urban forest. Int. Oaks, 30, 109–116. | Insect herbivory on urban trees: Complementary effects of tree neighbours and predation | Alex Stemmelen, Alain Paquette, Marie-Lise Benot, Yasmine Kadiri, Hervé Jactel, Bastien Castagneyrol | <p>Insect herbivory is an important component of forest ecosystems functioning and can affect tree growth and survival. Tree diversity is known to influence insect herbivory in natural forest, with most studies reporting a decrease in herbivory wi... |  | Biodiversity, Biological control, Community ecology, Ecosystem functioning, Herbivory | Ruth Arabelle Hufbauer | 2020-04-20 13:49:36 | View | |
03 Apr 2020
A macro-ecological approach to predators' functional responseMatthieu Barbier, Laurie Wojcik, Michel Loreau https://doi.org/10.1101/832220A meta-analysis to infer generic predator functional responseRecommended by Samir Simon Suweis based on reviews by Ludek Berec and gyorgy barabasSpecies interactions are classically derived from the law of mass action: the probability that, for example, a predation event occurs is proportional to the product of the density of the prey and predator species. In order to describe how predator and prey species populations grow, is then necessary to introduce functional response, describing the intake rate of a consumer as a function of food (e.g. prey) density. References [1] Volterra, V. (1928). Variations and Fluctuations of the Number of Individuals in Animal Species living together. ICES Journal of Marine Science, 3(1), 3–51. doi: 10.1093/icesjms/3.1.3 | A macro-ecological approach to predators' functional response | Matthieu Barbier, Laurie Wojcik, Michel Loreau | <p>Predation often deviates from the law of mass action: many micro- and meso-scale experiments have shown that consumption saturates with resource abundance, and decreases due to interference between consumers. But does this observation hold at m... |  | Community ecology, Food webs, Meta-analyses, Theoretical ecology | Samir Simon Suweis | 2019-11-08 15:42:16 | View | |
06 Mar 2020
Interplay between the paradox of enrichment and nutrient cycling in food websPierre Quévreux, Sébastien Barot and Élisa Thébault https://doi.org/10.1101/276592New insights into the role of nutrient cycling in food web dynamicsRecommended by Samraat Pawar based on reviews by Jean-François Arnoldi, Wojciech Uszko and 1 anonymous reviewer based on reviews by Jean-François Arnoldi, Wojciech Uszko and 1 anonymous reviewer
Understanding the factors that govern the relationship between structure, stability and functioning of food webs has been a central problem in ecology for many decades. Historically, apart from microbial and soil food webs, the role of nutrient cycling has largely been ignored in theoretical and empirical food web studies. A prime example of this is the widespread use of Lotka-Volterra type models in theoretical studies; these models per se are not designed to capture the effect of nutrients being released back into the system by interacting populations. Thus overall, we still lack a general understanding of how nutrient cycling affects food web dynamics. References [1] Quévreux, P., Barot, S. and E. Thébault (2020) Interplay between the paradox of enrichment and nutrient cycling in food webs. bioRxiv, 276592, ver. 7 peer-reviewed and recommended by PCI Ecology. doi: 10.1101/276592 | Interplay between the paradox of enrichment and nutrient cycling in food webs | Pierre Quévreux, Sébastien Barot and Élisa Thébault | <p>Nutrient cycling is fundamental to ecosystem functioning. Despite recent major advances in the understanding of complex food web dynamics, food web models have so far generally ignored nutrient cycling. However, nutrient cycling is expected to ... | Biodiversity, Community ecology, Ecosystem functioning, Food webs, Interaction networks, Theoretical ecology | Samraat Pawar | 2018-11-03 21:47:37 | View | ||
20 Feb 2023
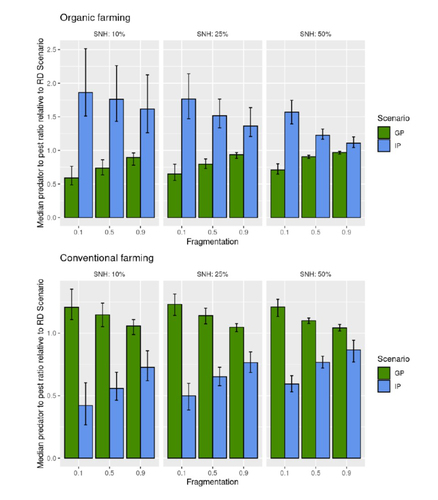
Best organic farming deployment scenarios for pest control: a modeling approachThomas Delattre, Mohamed-Mahmoud Memah, Pierre Franck, Pierre Valsesia, Claire Lavigne https://doi.org/10.1101/2022.05.31.494006Towards model-guided organic farming expansion for crop pest managementRecommended by Sandrine Charles based on reviews by Julia Astegiano, Lionel Hertzog and Sylvain Bart based on reviews by Julia Astegiano, Lionel Hertzog and Sylvain Bart
Reduce the impact the intensification of human activities has on the environmental is the challenge the humanity faces today, a major challenge that could be compared to climbing Everest without an oxygen supply. Indeed, over-population, pollution, burning fossil fuels, and deforestation are all evils which have had hugely detrimental effects on the environment such as climate change, soil erosion, poor air quality, and scarcity of drinking water to name but a few. In response to the ever-growing consumer demand, agriculture has intensified massively along with a drastic increase in the use of chemicals to ensure an adequate food supply while controlling crop pests. In this context, to address the disastrous effects of the intensive usage of pesticides on both human health and biodiversity, organic farming (OF) revealed as a miracle remedy with multiple benefits. Delattre et al. (2023) present a powerful modelling approach to decipher the crossed effects of the landscape structure and the OF expansion scenario on the pest abundance, both in organic and conventional (CF) crop fields. To this end, the authors ingeniously combined a grid-based landscape model with a spatially explicit predator-pest model. Based on an extensive in silico simulation process, they explore a diversity of landscape structures differing in their amount of semi-natural habitats (SHN) and in their fragmentation, to finally propose a ranking of various expansion scenarios according to the pest control methods in organic farming as well as to the pest and predators’ dissemination capacities. In total, 9 landscape structures (3 proportions of SHN x 3 fragmentation levels) were crossed with 3 expansion scenarios (RD = a random distribution of OF and CF in the grid; IP = isolated CF are converted; GP = CF within aggregates are converted), 4 pest management practices, 3 initial densities and 36 biological parameter combinations driving the predator’ and pest’s population dynamics. This exhaustive exploration of possible combinations of landscape and farming practices highlighted the main drivers of the various OF expansion scenarios, such as increased spillover of predators in isolated OF/CF fields, increased pest management efficiency in large patches of CF and the importance of the distance between OF and CF. In the end, this study brings to light the crucial role that landscape planning plays when OF practices have limited efficiency on pests. It also provides convincing arguments to the fact that converting to organic isolated CF as a priority seems to be the most promising scenario to limit pest densities in CF crops while improving predator to pest ratios (considered as a proxy of conservation biological control) in OF ones without increasing pest densities. Once further completed with model calibration validation based on observed life history traits data for both predators and pests, this work should be very helpful in sustaining policy makers to convince farmers of engaging in organic farming. REFERENCES Delattre T, Memah M-M, Franck P, Valsesia P, Lavigne C (2023) Best organic farming deployment scenarios for pest control: a modeling approach. bioRxiv, 2022.05.31.494006, ver. 2 peer-reviewed and recommended by Peer Community in Ecology. https://doi.org/10.1101/2022.05.31.494006 | Best organic farming deployment scenarios for pest control: a modeling approach | Thomas Delattre, Mohamed-Mahmoud Memah, Pierre Franck, Pierre Valsesia, Claire Lavigne | <p style="text-align: justify;">Organic Farming (OF) has been expanding recently around the world in response to growing consumer demand and as a response to environmental concerns. Its share of agricultural landscapes is expected to increase in t... |  | Agroecology, Biological control, Landscape ecology | Sandrine Charles | 2022-06-03 11:41:14 | View | |
31 May 2023
Conservation networks do not match the ecological requirements of amphibiansMatutini Florence, Jacques Baudry, Marie-Josée Fortin, Guillaume Pain, Joséphine Pithon https://doi.org/10.1101/2022.07.18.500425Amphibians under scrutiny - When human-dominated landscape mosaics are not in full compliance with their ecological requirementsRecommended by Sandrine Charles based on reviews by Peter Vermeiren and 1 anonymous reviewer based on reviews by Peter Vermeiren and 1 anonymous reviewer
Among vertebrates, amphibians are one of the most diverse groups with more than 7,000 known species. Amphibians occupy various ecosystems, including forests, wetlands, and freshwater habitats. Amphibians are known to be highly sensitive to changes in their environment, particularly to water quality and habitat degradation, so that monitoring abundance of amphibian populations can provide early warning signs of ecosystem disturbances that may also affect other organisms including humans (Bishop et al., 2012). Accordingly, efforts in habitat preservation and sustainable land and water management are necessary to safeguard amphibian populations. In this context, Matutini et al. (2023) compared ecological requirements of amphibian species with the quality of agricultural landscape mosaics. Doing so, they identified critical gaps in existing conservation tools that include protected areas, green infrastructures, and inventoried sites. Matutini et al. (2023) focused on nine amphibian species in the Pays-de-la-Loire region where the landscape has been fashioned over the years by human activities. Three of the chosen amphibian species are living in a dense hedgerow mosaic landscape, while five others are more generalists. Matutini et al. (2023) established multi-species habitat suitability maps, together with their levels of confidence, by combining single species maps with a probabilistic stacking method at 500-m resolution. From these maps, habitats were classified in five categories, from not suitable to highly suitable. Then, the circuit theory was used to map the potential connections between each highly suitable patch at the regional scale. Finally, comparing suitability maps with existing conservation tools, Matutini et al. (2023) were able to assess their coverage and efficiency. Whatever their species status (endangered or not), Matutini et al. (2023) highlighted some discrepancies between the ecological requirements of amphibians in terms of habitat quality and the conservation tools of the landscape mosaic within which they are evolving. More specifically, Matutini et al. (2023) found that protected areas and inventoried sites covered only a small proportion of highly suitable habitats, while green infrastructures covered around 50% of the potential habitat for amphibian species. Such a lack of coverage and efficiency of protected areas brings to light that geographical sites with amphibian conservation challenges are known but not protected. Regarding the landscape fragmentation, Matutini et al. (2023) found that generalist amphibian species have a more homogeneous distribution of suitable habitats at the regional scale. They also identified two bottlenecks between two areas of suitable habitats, a situation that could prove critical to amphibian movements if amphibians were forced to change habitats to global change. In conclusion, Matutini et al. (2023) bring convincing arguments in support of land-use species-conservation planning based on a better consideration of human-dominated landscape mosaics in full compliance with ecological requirements of the species that inhabit the regions concerned. ReferencesBishop, P.J., Angulo, A., Lewis, J.P., Moore, R.D., Rabb, G.B., Moreno, G., 2012. The Amphibian Extinction Crisis - what will it take to put the action into the Amphibian Conservation Action Plan? Sapiens - Surveys and Perspectives Integrating Environment and Society 5, 1–16. http://journals.openedition.org/sapiens/1406 Matutini, F., Baudry, J., Fortin, M.-J., Pain, G., Pithon, J., 2023. Conservation networks do not match ecological requirements of amphibians. bioRxiv, ver. 3 peer-reviewed and recommended by Peer Community in Ecology. https://doi.org/10.1101/2022.07.18.500425 | Conservation networks do not match the ecological requirements of amphibians | Matutini Florence, Jacques Baudry, Marie-Josée Fortin, Guillaume Pain, Joséphine Pithon | <p style="text-align: justify;">1. Amphibians are among the most threatened taxa as they are highly sensitive to habitat degradation and fragmentation. They are considered as model species to evaluate habitats quality in agricultural landscapes. I... | Biodiversity, Biogeography, Human impact, Landscape ecology, Macroecology, Spatial ecology, Metacommunities & Metapopulations, Species distributions, Terrestrial ecology | Sandrine Charles | 2022-09-20 14:40:03 | View | ||
23 Mar 2020

Intraspecific difference among herbivore lineages and their host-plant specialization drive the strength of trophic cascadesArnaud Sentis, Raphaël Bertram, Nathalie Dardenne, Jean-Christophe Simon, Alexandra Magro, Benoit Pujol, Etienne Danchin and Jean-Louis Hemptinne https://doi.org/10.1101/722140Tell me what you’ve eaten, I’ll tell you how much you’ll eat (and be eaten)Recommended by Sara Magalhães and Raul Costa-Pereira based on reviews by Bastien Castagneyrol and 1 anonymous reviewerTritrophic interactions have a central role in ecological theory and applications [1-3]. Particularly, systems comprised of plants, herbivores and predators have historically received wide attention given their ubiquity and economic importance [4]. Although ecologists have long aimed to understand the forces that govern alternating ecological effects at successive trophic levels [5], several key open questions remain (at least partially) unanswered [6]. In particular, the analysis of complex food webs has questioned whether ecosystems can be viewed as a series of trophic chains [7,8]. Moreover, whether systems are mostly controlled by top-down (trophic cascades) or bottom-up processes remains an open question [6]. References [1] Price, P. W., Bouton, C. E., Gross, P., McPheron, B. A., Thompson, J. N., & Weis, A. E. (1980). Interactions among three trophic levels: influence of plants on interactions between insect herbivores and natural enemies. Annual review of Ecology and Systematics, 11(1), 41-65. doi: 10.1146/annurev.es.11.110180.000353 | Intraspecific difference among herbivore lineages and their host-plant specialization drive the strength of trophic cascades | Arnaud Sentis, Raphaël Bertram, Nathalie Dardenne, Jean-Christophe Simon, Alexandra Magro, Benoit Pujol, Etienne Danchin and Jean-Louis Hemptinne | <p>Trophic cascades, the indirect effect of predators on non-adjacent lower trophic levels, are important drivers of the structure and dynamics of ecological communities. However, the influence of intraspecific trait variation on the strength of t... |  | Community ecology, Eco-evolutionary dynamics, Food webs, Population ecology | Sara Magalhães | 2019-08-02 09:11:03 | View | |
22 Mar 2021

Host-mediated, cross-generational intraspecific competition in a herbivore speciesBastien Castagneyrol, Inge van Halder, Yasmine Kadiri, Laura Schillé, Hervé Jactel https://doi.org/10.1101/2020.07.30.228544Plants preserve the ghost of competition past for herbivores, but mothers don’t careRecommended by Sara Magalhães based on reviews by Inês Fragata and Raul Costa-PereiraSome biological hypotheses are widely popular, so much so that we tend to forget their original lack of success. This is particularly true for hypotheses with catchy names. The ‘Ghost of competition past’ is part of the title of a paper by the great ecologist, JH Connell, one of the many losses of 2020 (Connell 1980). The hypothesis states that, even though we may not detect competition in current populations, their traits and distributions may be shaped by past competition events. Although this hypothesis has known a great success in the ecological literature, the original paper actually ends with “I will no longer be persuaded by such invoking of "the Ghost of Competition Past"”. Similarly, the hypothesis that mothers of herbivores choose host plants where their offspring will have a higher fitness was proposed by John Jaenike in 1978 (Jaenike 1978), and later coined the ‘mother knows best’ hypothesis. The hypothesis was readily questioned or dismissed: “Mother doesn't know best” (Courtney and Kibota 1990), or “Does mother know best?” (Valladares and Lawton 1991), but remains widely popular. It thus seems that catchy names (and the intuitive ideas behind them) have a heuristic value that is independent from the original persuasion in these ideas and the accumulation of evidence that followed it. The paper by Castagneryol et al. (2021) analyses the preference-performance relationship in the box tree moth (BTM) Cydalima perspectalis, after defoliation of their host plant, the box tree, by conspecifics. It thus has bearings on the two previously mentioned hypotheses. Specifically, they created an artificial population of potted box trees in a greenhouse, in which 60 trees were infested with BTM third instar larvae, whereas 61 were left uninfested. One week later, these larvae were removed and another three weeks later, they released adult BTM females and recorded their host choice by counting egg clutches laid by these females on the plants. Finally, they evaluated the effect of previously infested vs uninfested plants on BTM performance by measuring the weight of third instar larvae that had emerged from those eggs. This experimental design was adopted because BTM is a multivoltine species. When the second generation of BTM arrives, plants have been defoliated by the first generation and did not fully recover. Indeed, Castagneryol et al. (2021) found that larvae that developed on previously infested plants were much smaller than those developing on uninfested plants, and the same was true for the chrysalis that emerged from those larvae. This provides unequivocal evidence for the existence of a ghost of competition past in this system. However, the existence of this ghost still does not result in a change in the distribution of BTM, precisely because mothers do not know best: they lay as many eggs on plants previously infested than on uninfested plants. The demonstration that the previous presence of a competitor affects the performance of this herbivore species confirms that ghosts exist. However, whether this entails that previous (interspecific) competition shapes species distributions, as originally meant, remains an open question. Species phenology may play an important role in exposing organisms to the ghost, as this time-lagged competition may have been often overlooked. It is also relevant to try to understand why mothers don’t care in this, and other systems. One possibility is that they will have few opportunities to effectively choose in the real world, due to limited dispersal or to all plants being previously infested. References Castagneyrol, B., Halder, I. van, Kadiri, Y., Schillé, L. and Jactel, H. (2021) Host-mediated, cross-generational intraspecific competition in a herbivore species. bioRxiv, 2020.07.30.228544, ver. 5 peer-reviewed and recommended by PCI Ecology. doi: https://doi.org/10.1101/2020.07.30.228544 Connell, J. H. (1980). Diversity and the coevolution of competitors, or the ghost of competition past. Oikos, 131-138. doi: https://doi.org/10.2307/3544421 Courtney, S. P. and Kibota, T. T. (1990) in Insect-plant interactions (ed. Bernays, E.A.) 285-330. Jaenike, J. (1978). On optimal oviposition behavior in phytophagous insects. Theoretical population biology, 14(3), 350-356. doi: https://doi.org/10.1016/0040-5809(78)90012-6 Valladares, G., and Lawton, J. H. (1991). Host-plant selection in the holly leaf-miner: does mother know best?. The Journal of Animal Ecology, 227-240. doi: https://doi.org/10.2307/5456
| Host-mediated, cross-generational intraspecific competition in a herbivore species | Bastien Castagneyrol, Inge van Halder, Yasmine Kadiri, Laura Schillé, Hervé Jactel | <p>Conspecific insect herbivores co-occurring on the same host plant interact both directly through interference competition and indirectly through exploitative competition, plant-mediated interactions and enemy-mediated interactions. However, the... |  | Competition, Herbivory, Zoology | Sara Magalhães | 2020-08-03 15:50:23 | View | |
08 Jan 2020

Studies of NH4+ and NO3- uptake ability of subalpine plants and resource-use strategy identified by their functional traitsLegay Nicolas, Grassein Fabrice, Arnoldi Cindy, Segura Raphaël, Laîné Philippe, Lavorel Sandra, Clément Jean-Christophe https://doi.org/10.1101/372235Nitrate or not nitrate. That is the questionRecommended by Sébastien Barot based on reviews by Vincent Maire and 1 anonymous reviewerThe article by Legay et al. [1] addresses two main issues: the links between belowground and aboveground plant traits and the links between plant strategies (as defined by these traits) and the capacity to absorb nitrate and ammonium. I recommend this work because these are important and current issues. The literature on plant traits is extremely rich and the existence of a leaf economic spectrum linked to a gradient between conservative and acquisitive plants is now extremely well established [2-3]. Many teams are now working on belowground traits and possible links with the aboveground gradients [4-5]. It seems indeed that there is a root economic spectrum but this spectrum is apparently less pronounced than the leaf economic spectrum. The existence of links between the two spectrums are still controversial and are likely not universal as suggested by discrepant results and after all a plant could have a conservative strategy aboveground and an acquisitive strategy belowground (or vice-versa) because, indeed, constraints are different belowground and aboveground (for example because in given ecosystem/vegetation type light may be abundant but not water or mineral nutrients). The various results obtained also suggest that we do not full understand the diversity of belowground strategies, what is at stake with these strategies, and the links with root characteristics. References [1] Legay, N., Grassein, F., Arnoldi, C., Segura, R., Laîné, P., Lavorel, S. and Clément, J.-C. (2020). Studies of NH4+ and NO3- uptake ability of subalpine plants and resource-use strategy identified by their functional traits. bioRxiv, 372235, ver. 4 peer-reviewed and recommended by PCI Ecology. doi: 10.1101/372235 | Studies of NH4+ and NO3- uptake ability of subalpine plants and resource-use strategy identified by their functional traits | Legay Nicolas, Grassein Fabrice, Arnoldi Cindy, Segura Raphaël, Laîné Philippe, Lavorel Sandra, Clément Jean-Christophe | <p>The leaf economics spectrum (LES) is based on a suite of leaf traits related to plant functioning and ranges from resource-conservative to resource-acquisitive strategies. However, the relationships with root traits, and the associated belowgro... |  | Community ecology, Physiology, Terrestrial ecology | Sébastien Barot | 2018-07-19 14:22:28 | View | |
07 Oct 2019
Deer slow down litter decomposition by reducing litter quality in a temperate forestSimon Chollet, Morgane Maillard, Juliane Schorghuber, Sue Grayston, Jean-Louis Martin https://doi.org/10.1101/690032Disentangling effects of large herbivores on litter decompositionRecommended by Sébastien Barot based on reviews by 2 anonymous reviewersAboveground – belowground interactions is a fascinating field that has developed in ecology since about 20 years [1]. This field has been very fruitful as measured by the numerous articles published but also by the particular role it has played in the development of soil ecology. While soil ecology has for a long time developed partially independently from “general ecology” [2], the field of aboveground – belowground interactions has shown that all ecological interactions occurring within the soil are likely to impact plant growth and plant physiology because they have their roots within the soil. In turns, this should impact the aerial system of plants (higher or lower biomasses, changes in leaf quality…), which should cascade on the aboveground food web. Conversely, all ecological interactions occurring aboveground likely impact plant growth, which should cascade to their root systems, and thus to the soil functioning and the soil food web (through changes in the emission of exudates or inputs of dead roots…). Basically, plants are linking the belowground and aboveground worlds because, as terrestrial primary producers, they need to have (1) leaves to capture CO2 and exploit light and (2) roots to absorb water and mineral nutrients. The article I presently recommend [3] tackles this general issue through the prism of the impact of large herbivores on the decomposition of leaf litter. References [1] Hooper, D. U., Bignell, D. E., Brown, V. K., Brussard, L., Dangerfield, J. M., Wall, D. H. and Wolters, V. (2000). Interactions between Aboveground and Belowground Biodiversity in Terrestrial Ecosystems: Patterns, Mechanisms, and Feedbacks. BioScience, 50(12), 1049-1061. doi: 10.1641/0006-3568(2000)050[1049:ibaabb]2.0.co;2 | Deer slow down litter decomposition by reducing litter quality in a temperate forest | Simon Chollet, Morgane Maillard, Juliane Schorghuber, Sue Grayston, Jean-Louis Martin | <p>In temperate forest ecosystems, the role of deer in litter decomposition, a key nutrient cycling process, remains debated. Deer may modify the decomposition process by affecting plant cover and thus modifying litter abundance. They can also alt... | Community ecology, Ecosystem functioning, Herbivory, Soil ecology | Sébastien Barot | 2019-07-04 14:30:19 | View | ||
14 Dec 2022
The contrasted impacts of grasshoppers on soil microbial activities in function of primary production and herbivore dietSébastien Ibanez, Arnaud Foulquier, Charles Brun, Marie-Pascale Colace, Gabin Piton, Lionel Bernard, Christiane Gallet, Jean-Christophe Clément https://doi.org/10.1101/2022.07.04.497718Complex interactions between ecosystem productivity and herbivore diets lead to non-predicted effects on nutrient cyclingRecommended by Sébastien Barot based on reviews by Manuel Blouin and Tord Ranheim SveenThe authors present a study typical of the field of belowground-aboveground interactions [1]. This framework has been extremely fruitful since the beginning of 2000s [2]. It has also contributed to bridge the gap between soil ecology and the rest of ecology [3]. The study also pertains to the rich field on the impacts of herbivores on soil functioning [4]. The study more precisely tested during two years the effect on nutrient cycling of the interaction between the type of grassland (along a gradient of biomass productivity) and the diet of the community of insect herbivores (5 treatments manipulating the grasshopper community on 1 m2 plots, with a gradient from no grasshopper to grasshoppers either specialized on forbs or grasses). What seems extremely interesting is that the study is based on a rigorous hypothesis-testing approach. They compare the predictions of two frameworks: (1) The “productivity model” predicts that in productive ecosystems herbivores consume a high percentage of the net primary production thus accelerating nutrient cycling. (2) The “diet model” distinguishes herbivores consuming exploitative plants from those eating conservative plants. The former (later) type of herbivores favours conservative (exploitative) plants therefore decelerating (accelerating) nutrient cycling. Interestingly, the two frameworks have similar predictions (and symmetrically opposite predictions) in two cases out of four combinations between ecosystem productivities and types of diet (see Table 1). An other merit of the study is to combine in a rather comprehensive way all the necessary measurements to test these frameworks in combination: grasshopper diet, soil properties, characteristics of the soil microbial community, plant traits, vegetation survey and plant biomass. The results were in contradiction with the ‘‘diet model’’: microbial properties and nitrogen cycling did not depend on grasshopper diet. The productivity of the grasslands did impact nutrient cycling but not in the direction predicted by the “productivity model”: productive grasslands hosted exploitative plants that depleted N resources in the soil and microbes producing few extracellular enzymes, which led to a lower potential N mineralization and a deceleration of nutrient cycling. Because, the authors stuck to their original hypotheses (that were not confirmed), they were able to discuss in a very relevant way their results and to propose some interpretations, at least partially based on the time scales involved by the productivity and diet models. Beyond all the merits of this article, I think that two issues remain largely open in relation with the dynamics of the studied systems, and would deserve future research efforts. First, on the ‘‘short’’ term (up to several decades), can we predict how the communities of plants, soil microbes, and herbivores interact to drive the dynamics of the ecosystems? Second, at the evolutionary time scale, can we understand and predict the interactions between the evolution of plant, microbe and herbivore strategies and the consequences for the functioning of the grasslands? The two issues are difficult because of the multiple feedbacks involved. One way to go further would be to complement the empirical approach with models along existing research avenues [5, 6]. References [1] Ibanez S, Foulquier A, Brun C, Colace M-P, Piton G, Bernard L, Gallet C, Clément J-C (2022) The contrasted impacts of grasshoppers on soil microbial activities in function of primary production and herbivore diet. bioRxiv, 2022.07.04.497718, ver. 2 peer-reviewed and recommended by Peer Community in Ecology. https://doi.org/10.1101/2022.07.04.497718 [2] Hooper, D. U., Bignell, D. E., Brown, V. K., Brussaard, L., Dangerfield, J. M., Wall, D. H., Wardle, D. A., Coleman, D. C., Giller, K. E., Lavelle, P., Van der Putten, W. H., De Ruiter, P. C., et al. 2000. Interactions between aboveground and belowground biodiversity in terretrial ecosystems: patterns, mechanisms, and feedbacks. BioScience, 50, 1049-1061. https://doi.org/10.1641/0006-3568(2000)050[1049:IBAABB]2.0.CO;2 [3] Barot, S., Blouin, M., Fontaine, S., Jouquet, P., Lata, J.-C., and Mathieu, J. 2007. A tale of four stories: soil ecology, theory, evolution and the publication system. PLoS ONE, 2, e1248. https://doi.org/10.1371/journal.pone.0001248 [4] Bardgett, R. D., and Wardle, D. A. 2003. Herbivore-mediated linkages between aboveground and belowground communities. Ecology, 84, 2258-2268. https://doi.org/10.1890/02-0274 [5] Barot, S., Bornhofen, S., Loeuille, N., Perveen, N., Shahzad, T., and Fontaine, S. 2014. Nutrient enrichment and local competition influence the evolution of plant mineralization strategy, a modelling approach. J. Ecol., 102, 357-366. https://doi.org/10.1111/1365-2745.12200 [6] Schweitzer, J. A., Juric, I., van de Voorde, T. F. J., Clay, K., van der Putten, W. H., Bailey, J. K., and Fox, C. 2014. Are there evolutionary consequences of plant-soil feedbacks along soil gradients? Func. Ecol., 28, 55-64. https://doi.org/10.1111/1365-2435.12201
| The contrasted impacts of grasshoppers on soil microbial activities in function of primary production and herbivore diet | Sébastien Ibanez, Arnaud Foulquier, Charles Brun, Marie-Pascale Colace, Gabin Piton, Lionel Bernard, Christiane Gallet, Jean-Christophe Clément | <p style="text-align: justify;">Herbivory can have contrasted impacts on soil microbes and nutrient cycling, which has stimulated the development of conceptual frameworks exploring the links between below- and aboveground processes. The "productiv... | Ecosystem functioning, Herbivory, Soil ecology, Terrestrial ecology | Sébastien Barot | 2022-07-14 09:06:13 | View |
MANAGING BOARD
Julia Astegiano
Tim Coulson
Anna Eklof
Dominique Gravel
François Massol
Ben Phillips
Cyrille Violle










