
Marking invertebrates using RFID tags
 based on reviews by Simon Blanchet and 1 anonymous reviewer
based on reviews by Simon Blanchet and 1 anonymous reviewer
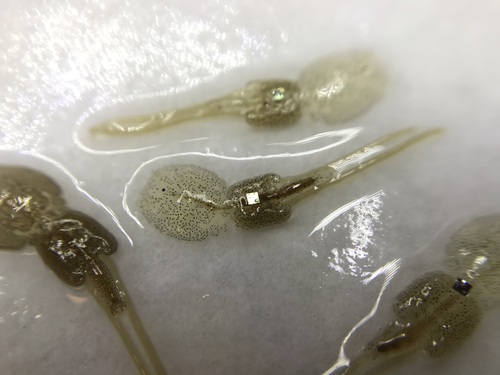
Methods for tagging an ectoparasite, the salmon louse Lepeophtheirus salmonis
Abstract
Recommendation: posted 12 January 2024, validated 12 January 2024
Schtickzelle, N. (2024) Marking invertebrates using RFID tags . Peer Community in Ecology, 100585. 10.24072/pci.ecology.100585
Recommendation
Guiding and monitoring the efficiency of conservation efforts needs robust scientific background information, of which one key element is estimating wildlife abundance and its spatial and temporal variation. As raw counts are by nature incomplete counts of a population, correcting for detectability is required (Clobert, 1995; Turlure et al., 2018). This can be done with Capture-Mark-Recapture protocols (Iijima, 2020). Techniques for marking individuals are diverse, e.g. writing on butterfly wings, banding birds, or using natural specific patterns in the individual’s body such as leopard fur or whale tail. Advancement in technology opens new opportunities for developing marking techniques, including strategies to limit mark identification errors (Burchill & Pavlic, 2019), and for using active marks that can transmit data remotely or be read automatically.
The details of such methodological developments frequently remain unpublished, the method being briefly described in studies that use it. For a few years, there has been however a renewed interest in proper publishing of methods for ecology and evolution. This study by Folk & Mennerat (2023) fits in this context, offering a nice example of detailed description and testing of a method to mark salmon ectoparasites using RFID tags. Such tags are extremely small, yet easy to use, even with automatic recording procedure. The study provides a very good basis protocol that should help researchers working for small species, in particular invertebrates. The study is complemented by a video illustrating the placement of the tag so the reader who would like to replicate the procedure can get a very precise idea of it.
References
Burchill, A. T., & Pavlic, T. P. (2019). Dude, where’s my mark? Creating robust animal identification schemes informed by communication theory. Animal Behaviour, 154, 203–208. https://doi.org/10.1016/j.anbehav.2019.05.013
Clobert, J. (1995). Capture-recapture and evolutionary ecology: A difficult wedding ? Journal of Applied Statistics, 22(5–6), 989–1008.
Folk, A., & Mennerat, A. (2023). Methods for tagging an ectoparasite, the salmon louse Lepeophtheirus salmonis (p. 2023.08.31.555695). bioRxiv, ver. 2 peer-reviewed and recommended by Peer Community in Ecology. https://doi.org/10.1101/2023.08.31.555695
Iijima, H. (2020). A Review of Wildlife Abundance Estimation Models: Comparison of Models for Correct Application. Mammal Study, 45(3), 177–188. https://doi.org/10.3106/ms2019-0082
Turlure, C., Pe’er, G., Baguette, M., & Schtickzelle, N. (2018). A simplified mark–release–recapture protocol to improve the cost effectiveness of repeated population size quantification. Methods in Ecology and Evolution, 9(3), 645–656. https://doi.org/10.1111/2041-210X.12900
The recommender in charge of the evaluation of the article and the reviewers declared that they have no conflict of interest (as defined in the code of conduct of PCI) with the authors or with the content of the article. The authors declared that they comply with the PCI rule of having no financial conflicts of interest in relation to the content of the article.
Research Council of Norway grant nr 287405
Evaluation round #1
DOI or URL of the preprint: https://doi.org/10.1101/2023.08.31.555695
Version of the preprint: 1
Author's Reply, 20 Dec 2023
Decision by Nicolas Schtickzelle , posted 30 Oct 2023, validated 30 Oct 2023
, posted 30 Oct 2023, validated 30 Oct 2023
Dear authors,
your manuscript has been reviewed by two colleagues who made a series of constructive comments ro further improve it.
Methodology is not always particularly well considered as worth publishing in ecology and evolution, a view I strongly disagree with because established methodologies are the base of the principle that science is based on replication. In that sense, your manuscript is very interesting. I agree with one reviewer that it might however benefit from elaborating on key questions this approach can help studying and whether the methodology might be applied to a broader range of species.
One reviewer especially makes details suggestions about methodological aspects. I generally agree with them and I'm convinced that your manuscript could be improved by taking them into account, modifying some analyses or adding some words of discussion for aspects you cannot change (e.g. using a single tank per glue type).
Extra minor comments:
(133) remove the unecessary "to" before "2-octyl"
(342) I guess by "relies on fine motor skills" you mean that the experimenter needs to display some movement precision for the tagging to be performed adequately. Judging from the video, I guess it is likely possible to define a protocol that would ease this, e.g. by placing the dish on the table, using a magnifier... However, it's hard to determine if the precision needed is likely possessed by many individuals or only a few. Maybe a few words to precise what you mean by "relies on fine motor skills" would be useful.
I look forward to reading your revised version to be considered for recommendation.
Best regards,
Nicolas
Reviewed by Simon Blanchet, 12 Oct 2023
Dear Authors
I have now read your MS "Methods for tagging an ectoparasite, the salmon louse Lepeophtheirus salmonis". The MS is very well written and it describes a new method for tagging fish ectoparasite. I don't have major criticisms as most experiments have been seriously performed, as well as associted statistical tests. I was a bit surprised that mortality and fecundity were not compared between a tagged and an untagged group (although authors provide an explanation that was not super convincing to me). This is the only methodological limitation I can see, and I suggest authors to discuss this briefly, or perhaps they have personnal observation that may be included into the Discussion to convince readers that mortality (to a lesser extent fecundity as the test for this parameter is more solid) is not (strongly) different between tagged and untagged groups. In addition, I was a bit frustrated not to read a bit more about the research avenues that are now opened thanks to this method. I would like you to elaborate a bit on what are the key scientific questions that can now be tackled (in salmon lice and other fish ectoparasites), and to which extent you think this tagging approach can be extended to other (fish or not) ectoparasite and other invertebrates.
Minor comments:
l. 55-56: I think there are also good examples of individual tagging in butterflies (writing on wings). Please add references if you find some.
Figure 3: I would advice starting the y-axis to zero; as it is it seems like retention rate drop to 0 whereas it actually drops to 0.3
Discussion first paragraph: please indicate that a specific toxicity test would be required to tease apart the two hypotheses (tank effect or toxicity). If the agent is toxic this may be problematic for further studies.










