
Catching the fly in dystopian times
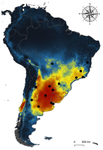
Environmental variables determining the distribution of an avian parasite: the case of the Philornis torquans complex (Diptera: Muscidae) in South America
Abstract
Recommendation: posted 17 March 2020, validated 30 March 2020
Medel, R. (2020) Catching the fly in dystopian times. Peer Community in Ecology, 100049. https://doi.org/10.24072/pci.ecology.100049
Recommendation
Host-parasite interactions are ubiquitous on Earth. They are present in almost every conceivable ecosystem and often result from a long history of antagonist coevolution [1,2]. Recent studies on climate change have revealed, however, that modification of abiotic variables are often accompanied by shifts in the distributional range of parasites to habitats far beyond their original geographical distribution, creating new interactions in novel habitats with unpredictable consequences for host community structure and organization [3,4]. This situation may be especially critical for endangered host species having small population abundance and restricted distribution range. The infestation of bird species with larvae of the muscid fly genus Philornis is a case in point. At least 250 bird species inhabiting mostly Central and South America are infected by Philornis flies [5,6]. Fly larval development occurs in bird faeces, nesting material, or inside nestlings, affecting the development and nestling survival.
Recent reports indicate significant reduction of bird numbers associated with recent Philornis infection, the most conspicuous being Galapagos finches [7,8]. One way to prevent this potential effect consists in to examine the expected geographical shift of Philornis fly species under future climate change scenarios so that anticipatory conservation practices become implemented for endangered bird species. In this regard, Ecological Niche Modeling (ENM) techniques have been increasingly used as a useful tool to predict disease transmission as well as the species becoming infected under different climate change scenarios [9-11]. The paper of Cuervo et al. [12] is an important advance in this regard. By identifying for the first time the macro-environmental variables influencing the abiotic niche of species of the Philornis torquans complex in southern South America, the authors perform a geographical projection model that permits identification of the areas susceptible to be colonized by Philornis species in Argentina, Brazil, and Chile, including habitats where the parasitic fly is still largely absent at present. Their results are promissory for conservation studies and contribute to the still underdeveloped issue of the way climate change impacts on antagonistic ecological relationships.
References
[1] Thompson JN (1994) The Coevolutionary Process. University of Chicago Press.
[2] Poulin R (2007) Evolutionary Ecology of Parasites: (Second Edition). Princeton University Press. doi: 10.2307/j.ctt7sn0x
[3] Pickles RSA, Thornton D, Feldman R, Marques A, Murray DL (2013) Predicting shifts in parasite distribution with climate change: a multitrophic level approach. Global Change Biology, 19, 2645–2654. doi: 10.1111/gcb.12255
[4] Marcogliese DJ (2016) The distribution and abundance of parasites in aquatic ecosystems in a changing climate: More than just temperature. Integrative and Comparative Biology, 56, 611–619. doi: 10.1093/icb/icw036
[5] Dudaniec RY, Kleindorfer S (2006) Effects of the parasitic flies of the genus Philornis (Diptera: Muscidae) on birds. Emu - Austral Ornithology, 106, 13–20. doi: 10.1071/MU04040
[6] Antoniazzi LR, Manzoli DE, Rohrmann D, Saravia MJ, Silvestri L, Beldomenico PM (2011) Climate variability affects the impact of parasitic flies on Argentinean forest birds. Journal of Zoology, 283, 126–134. doi: 10.1111/j.1469-7998.2010.00753.x
[7] Fessl B, Sinclair BJ, Kleindorfer S (2006) The life-cycle of Philornis downsi (Diptera: Muscidae) parasitizing Darwin’s finches and its impacts on nestling survival. Parasitology, 133, 739–747. doi: 10.1017/S0031182006001089
[8] Kleindorfer S, Peters KJ, Custance G, Dudaniec RY, O’Connor JA (2014) Changes in Philornis infestation behavior threaten Darwin’s finch survival. Current Zoology, 60, 542–550. doi: 10.1093/czoolo/60.4.542
[9] Johnson EE, Escobar LE, Zambrana-Torrelio C (2019) An ecological framework for modeling the geography of disease transmission. Trends in Ecology and Evolution, 34, 655–668. doi: 10.1016/j.tree.2019.03.004
[10] Carvalho BM, Rangel EF, Ready PD, Vale MM (2015) Ecological niche modelling predicts southward expansion of Lutzomyia (Nyssomyia) flaviscutellata (Diptera: Psychodidae: Phlebotominae), vector of Leishmania (Leishmania) amazonensis in South America, under climate change. PLOS ONE, 10, e0143282. doi: 10.1371/journal.pone.0143282
[11] Garrido R, Bacigalupo A, Peña-Gómez F, Bustamante RO, Cattan PE, Gorla DE, Botto-Mahan C (2019) Potential impact of climate change on the geographical distribution of two wild vectors of Chagas disease in Chile: Mepraia spinolai and Mepraia gajardoi. Parasites and Vectors, 12, 478. doi: 10.1186/s13071-019-3744-9
[12] Cuervo PF, Percara A, Monje L, Beldomenico PM, Quiroga MA (2020) Environmental variables determining the distribution of an avian parasite: the case of the Philornis torquans complex (Diptera: Muscidae) in South America. bioRxiv, 839589, ver. 5 peer-reviewed and recommended by PCI Ecology. doi: 10.1101/839589
The recommender in charge of the evaluation of the article and the reviewers declared that they have no conflict of interest (as defined in the code of conduct of PCI) with the authors or with the content of the article. The authors declared that they comply with the PCI rule of having no financial conflicts of interest in relation to the content of the article.
Evaluation round #1
DOI or URL of the preprint: https://doi.org/10.1101/839589
Version of the preprint: 1
Author's Reply, 05 Mar 2020
Decision by Rodrigo Medel, posted 13 Jan 2020
Dear Dr. Cuervo and Dr. Quiroga,
I received three reviews of your paper entitled "Experimental variables determining the presence of an avian parasite: the case of the Philornis torquans complex (Diptera: Muscidae) in South America". After checking their comments, it is perfectly clear they agree this is an interesting contribution that will make an important contribution to the literature on parasitic flies-bird relationships. However, they also pointed out a number of issues that need to be addressed before accepting your contribution. You can check directly their pdfs for specific recommendations. However, I would like you to consider especially the following observations.
- A clear sentence in the Introduction section that provides a justification to carry out this study is needed. Likewise, a sentence at the end that provides a strong take-home message.
- Consider (if the major aim of this ms basis on conservation) a sentence on how the results of this work may contribute to conservation.
- Consider to include additional information on the host range of P. torquatus (if available).
- Please include information on the extent to which the distribution of host species accounts for the geographic distribution of P. torquans. In the absence of information you can include a sentence discussing this issue.
- Discuss whether environmental factors act directly on P. torquans distribution or indirectly, through bird hosts as host availability may determine parasite distribution by itself.
- Include information regarding the way locations were chosen (random?, from the available literature?). This seems to be a sensitive issue because of a potential spatial autocorrelation of study sites.
- Did you examine the sensitivity of model accuracy to the sample size used?
- Please consider (or explain) the issue that only 3 out of 900 models met the criteria for inclusion. Did you correct for multiple tests?
- Consider to reduce the discussion section (paragraphs 366-389) may be collapsed to make the point more clear.
- Consider including Figs S1 and S2 in the main text. Fig 3c needs a better legend.
- Please include in the Discussion section or supplementary material a list of the potential or current bird species affected by the fly complex.
I look forward receiving you revised manuscript.
Best regards
Rodrigo Medel
Reviewed by anonymous reviewer 1, 20 Jan 2020
Reviewed by anonymous reviewer 4, 22 Dec 2019
This is an interesting application of an analytical approach to understand and predict the distribution of Philornis spp. in South America. I have two minor comments:
1) In the Materials and Methods section ('Species occurrence data'), the authors state data was obtained from field surveys conducted in Argentina but do not provide any details pertaining to these surveys (when, where, etc).
2) In the Materials and Methods section, under 'Environmental data and selection of variables', it is unclear what 10' means ("We coarsed environmental data to 10’ (~17 × ~17 km) spatial resolution, which approximately matches the uncertainty inherent in the occurrence data.")
Reviewed by anonymous reviewer 2, 31 Dec 2019
Reviewed by anonymous reviewer 3, 17 Dec 2019
Dear Editor
I pleased to present you the review of manuscript entitled “Environmental variables determining the presence of an avian parasite: the case of the Philornis torquans complex (Diptera: Muscidae) in South America” of Cuervo et al. This study pretends provide a methodological framework to understand the potential distribution of Philornis complex, a parasitic fly group of birds. The manuscript is clear in their methodology (with exceptions, see general comments) and results and could be used as a powerful tool to predict the distribution of this flies along the distribution known of this complex and also, to conserve endangered birds. However, need a major review in some aspects. I recommend publish this manuscript when the questions are resolved by the authors.
General comments:
I detect four main problems
1. In Methodology, the authors not mentioned as we obtained their dataset. This is very important because is the baseline for any modelling niche study. I necessary that the author mention how sites were surveyed? how many replicates (or pseudoreplicates) have any sites? Seasonality? How standardized the information? Taxonomic authority that classify the philornis? Methodology…. Etc, etc… is completely necessary read this information.
2. The low amount of records not autocorrelated. This said me that all previous sampled were realized in closer sites. Although the authors adequately detail each step carried out through the recommendations of other manuscripts, I wish they could at least include in the discussion of the work somewhat more elaborated with respect to the predictions of the model with a larger number of data from the Philornis torquans complex.
3. In the methodology, the rationale for using this fly complex is because it affects only one threatened bird species. The authors could provide in the supplementary material and then in the discussion a potential or actual list of birds affected by this fly complex.
4. Shorten and rephrase part of the discussion. Please, take into account some of the suggestions written here take.
In particular, I have many other recommendations:
L56-58. Reorder! First taxonomy and then, reference!
L62. Please, clarify this. In the first sentences you tell me that there three genera, including Philornis generating Myasis. But then, you tell me that the larvae of Philornis are coprophagous, semi-haematophagous and subcutaneous. So, what type of feeding is myasis?
L70-71. How depend? It is obvious. Change the magnitude? You said this in the preceding sentence. Change the intensity? You don’t said the prevalence. Please clarify or remove and reinforce the previous sentence.
L72. Reference after “…negligible”
L84-89. Please separate in two phrases.
L113. There is a problem here. The complex is choose because affect to yellow cardinal only? Or affect other endangered birds? Please add new examples or number of bird affected with respective references.
L118-121. I need more information. How field surveys? Where? How many replicates por site? In what season your sampled? What literature you consulted? Number of references? How many nest were reviewed in each site? … please, provided ALL information that support the obtained dataset.
L.119-120 who determined the larvae? The authors used taxonomic key? Molecular depositories? Please provide these data.
L136-138. I am not convinced by using only 18 data to model the presence of Philornis. When I have worked in modeling, we have always been asked in journals for a number not less than 40 records and especially records that are not autocorrelated. I understand that sampling generates a bias. Questions: did you test the model with the 80 initial records, regardless of whether there is autocorrelation? Did you generate the model once you only considered 34 sites? My idea is that you test these models to see how substantive is the change between the initial model versus the clean version.
L201-202. Please, also provide a negative argument to small dataset. What level of precision is obtained whit few data vs large dataset?
L206. Some reference?
L 209-211. Labud et al. 2003 show data about the movement? I don’t think so. Contrarily, Showler & Osbrink 2015 efectively show movement >13 km in some cases. Please provide information about Philornis species that you use for modelling.
L250-251. Careful! The comparison that you mention has been studied in Philornis species? Do you have information about physiological curve of thermal tolerance? Metabolic exchange? Temperature stress o resistance? Thermal limits? Hypoxia? Provide any evidence about this comparison!
L253-256. Remove this and incorporate in the legend of the figure!
L301. Figure “3a” change capital letter and number
L336-342. Please, provide a brief sentences mention that could happen with a high number of records not autocorrelated? The model should be the same?
L343-344. You not mentioned how obtain the primary dataset. This is completely necessary for any modelling niche! Please provide all information in the Methodology section and subsections.
L349. Migrate is the same of movement? Please clarify this because the torquans complex move of some way. How move by day? By year? There is literature?
L350 P. downsi inhabits in Galápagos! That species are limited by the sea! In your case torquans complex is not limited for geographical barriers!if the authos don’t suspect to Philornis change among states is necessary provide a explain to the potential movement.
L362-364. Along the latitudes is possible that torquans complex present reaction norm of its physiological minimum thermal temperature? Please provide a short sentence with some example or hypothesis please.
L367-370. Mention species, provide references please
L376. Cursive Protocalliphora
L375-378. Some redundant with the previous sentence. Please, shorten the sentence and this paragraph.
L381-382. How many time live a Philornis? There is some reference? Life table?
L401-406. In global warming scenario, how affect this to your results? Do you thinks tha could increase the infestation? The reproduction increase with the temperature? What other fitness traits increase/decrease with high temperatures?
L409-411. This must be mentioned before in the methodology!
L443-446. I thinks that this could develop more! Would it be possible for Philornis torquans complex to invade Chile through its own mechanisms? certainly, the authors do not have this clear, since they do not know the capacity of movement (or migration) of the complex as well as physiological aspects that could give a better explanation to the invasion in an area of Chile where average temperatures could ensure adequate development of the species.










