

 based on reviews by 2 anonymous reviewers
based on reviews by 2 anonymous reviewers
Dispersal is a key element of the natural dynamics of meta-communities, and plays a central role in the success of populations colonizing new landscapes. Understanding how demographic processes may affect the speed at which alien species spread through environmentally-heterogeneous habitat fragments is therefore of key importance to manage biological invasions. This requires studying together the complex interplay of dispersal and population processes, two inextricably related phenomena that can produce many possible outcomes. Stochastic models offer an opportunity to describe this kind of process in a meaningful way, but to ensure that they are realistic (sensu Levins 1966) it is also necessary to combine model simulations with empirical data (Snäll et al. 2007).
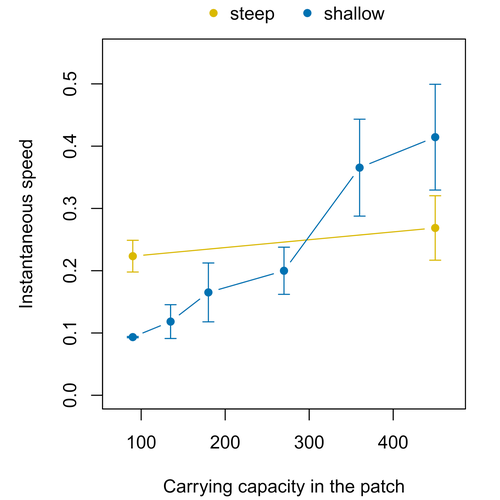
Morel-Journel et al. (2023) put together stochastic models and experimental data to study how population density may affect the speed at which alien species spread through a heterogeneous landscape. They do it by focusing on what they call ‘colonisation debt’, which is merely the impact that population density at the invasion front may have on the speed at which the species colonizes patches of different carrying capacities. They investigate this issue through two largely independent approaches. First, a stochastic model of dispersal throughout the patches of a linear, 1-dimensional landscape, which accounts for different degrees of density-dependent growth. And second, a microcosm experiment of a parasitoid wasp colonizing patches with different numbers of host eggs. In both cases, they compare the velocity of colonization of patches with lower or higher carrying capacity than the previous one (i.e. what they call upward or downward gradients).
Their results show that density-dependent processes influence the speed at which new fragments are colonized is significantly reduced by positive density dependence. When either population growth or dispersal rate depend on density, colonisation debt limits the speed of invasion, which turns out to be dependent on the strength and direction of the gradient between the conditions of the invasion front, and the newly colonized patches. Although this result may be quite important to understand the meta-population dynamics of dispersing species, it is important to note that in their study the environmental differences between patches do not take into account eventual shifts in the scenopoetic conditions (i.e. the values of the environmental parameters to which species niches’ respond to; Hutchinson 1978, see also Soberón 2007). Rather, differences arise from variations in the carrying capacity of the patches that are consecutively invaded, both in the in silico and microcosm experiments. That is, they account for potential differences in the size or quality of the invaded fragments, but not on the costs of colonizing fragments with different environmental conditions, which may also determine invasion speed through niche-driven processes. This aspect can be of particular importance in biological invasions or under climate change-driven range shifts, when adaptation to new environments is often required (Sakai et al. 2001; Whitney & Gabler 2008; Hill et al. 2011).
The expansion of geographical distribution ranges is the result of complex eco-evolutionary processes where meta-community dynamics and niche shifts interact in a novel physical space and/or environment (see, e.g., Mestre et al. 2020). Here, the invasibility of native communities is determined by niche variations and how similar are the traits of alien and native species (Hui et al. 2023). Within this context, density-dependent processes will build upon and heterogeneous matrix of native communities and environments (Tischendorf et al. 2005), to eventually determine invasion success. What the results of Morel-Journel et al. (2023) show is that, when the invader shows density dependence, the invasion process can be slowed down by variations in the carrying capacity of patches along the dispersal front. This can be particularly useful to manage biological invasions; ongoing invasions can be at least partially controlled by manipulating the size or quality of the patches that are most adequate to the invader, controlling host populations to reduce carrying capacity. But further, landscape manipulation of such kind could be used in a preventive way, to account in advance for the effects of the introduction of alien species for agricultural exploitation or biological control, thereby providing an additional safeguard to practices such as the introduction of parasitoids to control plagues. These practical aspects are certainly worth exploring further, together with a more explicit account of the influence of the abiotic conditions and the characteristics of the invaded communities on the success and speed of biological invasions.
REFERENCES
Hill, J.K., Griffiths, H.M. & Thomas, C.D. (2011) Climate change and evolutionary adaptations at species' range margins. Annual Review of Entomology, 56, 143-159. https://doi.org/10.1146/annurev-ento-120709-144746
Hui, C., Pyšek, P. & Richardson, D.M. (2023) Disentangling the relationships among abundance, invasiveness and invasibility in trait space. npj Biodiversity, 2, 13. https://doi.org/10.1038/s44185-023-00019-1
Hutchinson, G.E. (1978) An introduction to population biology. Yale University Press, New Haven, CT.
Levins, R. (1966) The strategy of model building in population biology. American Scientist, 54, 421-431.
Mestre, A., Poulin, R. & Hortal, J. (2020) A niche perspective on the range expansion of symbionts. Biological Reviews, 95, 491-516. https://doi.org/10.1111/brv.12574
Morel-Journel, T., Haond, M., Duan, L., Mailleret, L. & Vercken, E. (2023) Colonisation debt: when invasion history impacts current range expansion. bioRxiv, 2022.11.13.516255, ver. 3 peer-reviewed and recommended by Peer Community in Ecology. https://doi.org/10.1101/2022.11.13.516255
Snäll, T., B. O'Hara, R. & Arjas, E. (2007) A mathematical and statistical framework for modelling dispersal. Oikos, 116, 1037-1050. https://doi.org/10.1111/j.0030-1299.2007.15604.x
Sakai, A.K., Allendorf, F.W., Holt, J.S., Lodge, D.M., Molofsky, J., With, K.A., Baughman, S., Cabin, R.J., Cohen, J.E., Ellstrand, N.C., McCauley, D.E., O'Neil, P., Parker, I.M., Thompson, J.N. & Weller, S.G. (2001) The population biology of invasive species. Annual Review of Ecology and Systematics, 32, 305-332. https://doi.org/10.1146/annurev.ecolsys.32.081501.114037
Soberón, J. (2007) Grinnellian and Eltonian niches and geographic distributions of species. Ecology Letters, 10, 1115-1123. https://doi.org/10.1111/j.1461-0248.2007.01107.x
Tischendorf, L., Grez, A., Zaviezo, T. & Fahrig, L. (2005) Mechanisms affecting population density in fragmented habitat. Ecology and Society, 10, 7. https://doi.org/10.5751/ES-01265-100107
Whitney, K.D. & Gabler, C.A. (2008) Rapid evolution in introduced species, 'invasive traits' and recipient communities: challenges for predicting invasive potential. Diversity and Distributions, 14, 569-580. https://doi.org/10.1111/j.1472-4642.2008.00473.x
Overall, the revisions to the manuscript and the authors' response letter addresses many of the concerns I had. The authors have more clearly defined the gradients they use in their study, justified choices in their design that were not originally clear and better integrated their experimental and simulation results. I remain a bit apprehensive about the role of gradients vs. periodicity of the environment, but feel the additional simulations do make it more clear how each of these factors contribute to the colonisation debt in their model.
After checking the authors' reply, I consider that they did a good job in addressing all the issues and concerns pointed out by us.
DOI or URL of the preprint: https://doi.org/10.1101/2022.11.13.516255
Version of the preprint: 2
 , posted 24 Jan 2023, validated 24 Jan 2023
, posted 24 Jan 2023, validated 24 Jan 2023Dear authors
Two anonymous peers have reviewed your article. Both believe that your model is sound, and timely, but both highlight that your results are mostly limited to the effects of density-dependent processes during the invasion. In this regard, I was expecting a more explicit treatment of environmental gradients, like one of the reviewers. It would be great if your model included the need for shifts in the environmental conditions used by the species that are common in range margins and/or during invasions. I now understand that this goes beyond the model you are presenting here, but I encourage you to pursue this objective further in the future.
Both reviewers provide constructive and useful insights to improve your analyses, and in particular the discussion. Before recommending this paper I would like you to follow their advice, or alternatively provide solid and convincing arguments not to do so. While reviewing your paper please bear in mind that I tend agree with one the reviewers ing that your in silico experiments should be backed up by analyses without the descending gradients, to tease apart the role of these gradients when carrying capacity is low. And also that the way you classify gradients according to carrying capacity is potentially confusing.
I hope these comments are useful to improve your work. Should youd decide to resubmit this manuscript, please provide a detailed answer to both reviewers' comments. I'm looking forward to see that new version.
All the best,
Joaquín