
YOCCOZ Nigel
- Arctic and Marine Biology, UiT The Arctic University of Norway, Tromsø, Norway
- Biodiversity, Biological invasions, Climate change, Demography, Food webs, Population ecology, Species distributions, Statistical ecology
- recommender
Recommendations: 3
Reviews: 3
Recommendations: 3
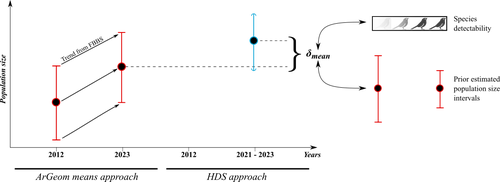
Reassessment of French breeding bird population sizes using citizen science and accounting for species detectability
Reassessment of French breeding bird population sizes: from citizen science observations to nationwide estimates
Recommended by Nigel Yoccoz based on reviews by 2 anonymous reviewersEstimating populations size of widespread, common species in a relatively large and heterogeneous country like France is difficult for several reasons, from having a sample covering well the diverse ecological gradients to accounting for detectability, the fact that absence of a species may represent a false negative, the species being present but not detected. Bird communities have been the focus of a very large number of studies, with some countries like the UK having long traditions of monitoring both common and rare species. Nabias et al. use a large, structured citizen science project to provide new estimates of common bird species, accounting for detectability and using different habitat and climate covariates to extrapolate abundance to non-sampled areas. About 2/3 of the species had estimates higher than what would have been expected using a previous attempt at estimating population size based in part on expert knowledge and projected using estimates of trends to the period covered by the citizen science sampling. Some species showed large differences between the two estimates, which could be in part explained by accounting for detectability.
This paper uses what is called model-based inference (as opposed to design-based inference, that uses the design to make inferences about the whole population; Buckland et al. 2000), both in terms of detectability and habitat suitability. The estimates obtained depend on how well the model components approximate the underlying processes, which in a complex dataset like this one is not easy to assess. But it clearly shows that detectability may have substantial implications for the population size estimates. This is of course not new but has rarely been done at this scale and using a large sample obtained on many species. Interesting further work could focus on testing the robustness of the model-based approach by for example sampling new plots and compare the expected values to the observed values. Such a sampling could be stratified to maximize the discrimination between expected low and high abundances, at least for species where the estimates might be considered as uncertain, or for which estimating population sizes is deemed important.
References
Buckland, S. T., Goudie, I. B. J., & Borchers, D. L. (2000). Wildlife Population Assessment: Past Developments and Future Directions. Biometrics, 56(1), 1-12. https://doi.org/10.1111/j.0006-341X.2000.00001.x
Nabias, J., Barbaro, L., Fontaine, B., Dupuy, J., Couzi, L., et al. (2024) Reassessment of French breeding bird population sizes using citizen science and accounting for species detectability. HAL, ver. 2 peer-reviewed and recommended by Peer Community in Ecology. https://hal.science/hal-04478371
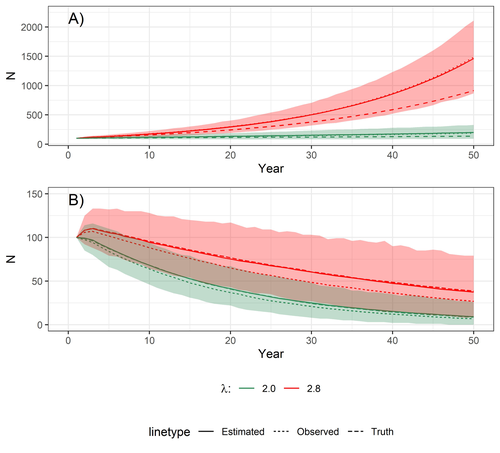
Accounting for observation biases associated with counts of young when estimating fecundity: case study on the arboreal-nesting red kite (Milvus milvus)
Accounting for observation biases associated with counts of young: you may count too many or too few...
Recommended by Nigel Yoccoz based on reviews by Steffen Oppel and 1 anonymous reviewerMost species are hard to observe, and different methods are required to estimate demographic parameters such as the number of young individuals produced (one measure of breeding success) and survival. In the former case, and in particular for birds of prey, it often relies upon direct observations of breeding pairs on their nests. Two problems can then occur, that some young are missed and therefore the breeding success is underestimated (“false negatives”), but it is also possible that because for example of the nest structure or vegetation surrounding the nest, more young birds than in fact are present are counted (“false positives”). Sollmann et al. (2024) address this problem by using data where the truth is known as each nest was also accessed after climbing the tree, and a hierarchical model accounting for both undercounts and overcounts. Finally, they assess the impact of this correction on projected population size using simulations.
This paper is a solid contribution to the panoply of methods and models that are available for monitoring populations, and has potential applications for many species for which both false positives and false negatives can be a problem. The results on the projected population sizes – showing that for growing populations correcting for bias can lead to large differences in population sizes after a few decades – may seem counterintuitive as population growth rate of long-lived species such as birds of prey is not very sensitive to a change in breeding success (as compared to adult survival). However, one should just be reminded that a small difference in population growth rate may translate to a large difference after many years – for example a growth rate of 1.05 after 50 years mean than population size is multiplied by 11.5, whereas a growth of 1.03 after 50 years mean a multiplication by 4.4, more than twice less individuals. Small differences may matter a lot if they are sustained, and a key aspect of management is to ensure that they are. Of course, management actions having an impact on survival may be more effective, but they might be harder to achieve than for example ensuring that birds of prey breed successfully.
References
Sollmann Rahel, Adenot Nathalie, Spakovszky Péter, Windt Jendrik, Mattsson Brady J. 2024. Accounting for observation biases associated with counts of young when estimating fecundity. bioRxiv, v. 2 peer-reviewed and recommended by Peer Community in Ecology. https://doi.org/10.1101/2023.12.01.569571
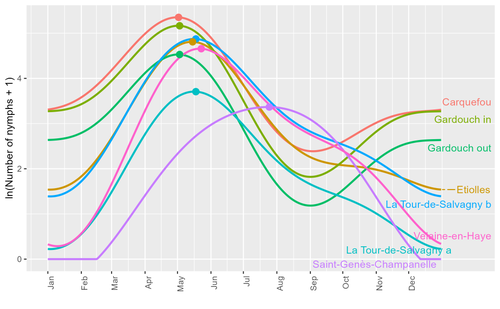
Seasonality of host-seeking Ixodes ricinus nymph abundance in relation to climate
Assessing seasonality of tick abundance in different climatic regions
Recommended by Nigel Yoccoz based on reviews by 2 anonymous reviewersTick-borne pathogens are considered as one of the major threats to public health – Lyme borreliosis being a well-known example of such disease. Global change – from climate change to changes in land use or invasive species – is playing a role in the increased risk associated with these pathogens. An important aspect of our knowledge of ticks and their associated pathogens is seasonality – one component being the phenology of within-year tick occurrences. This is important both in terms of health risk – e.g., when is the risk of encountering ticks high – and ecological understanding, as tick dynamics may depend on the weather as well as different hosts with their own dynamics and habitat use.
Hoch et al. (2023) provide a detailed data set on the phenology of one species of tick, Ixodes ricinus, in 6 different locations of France. Whereas relatively cool sites showed a clear peak in spring-summer, warmer sites showed in addition relatively high occurrences in fall-winter, with a minimum in late summer-early fall. Such results add robust data to the existing evidence of the importance of local climatic patterns for explaining tick phenology.
Recent analyses have shown that the phenology of Lyme borreliosis has been changing in northern Europe in the last 25 years, with seasonal peaks in cases occurring now 6 weeks earlier (Goren et al. 2023). The study by Hoch et al. (2023) is of too short duration to establish temporal changes in phenology (“only” 8 years, 2014-2021, see also Wongnak et al 2021 for some additional analyses; given the high year-to-year variability in weather, establishing phenological changes often require longer time series), and further work is needed to get better estimates of these changes and relate them to climate, land-use, and host density changes. Moreover, the phenology of ticks may also be related to species-specific tick phenology, and different tick species do not respond to current changes in identical ways (see for example differences between the two Ixodes species in Finland; Uusitalo et al. 2022). An efficient surveillance system requires therefore an adaptive monitoring design with regard to the tick species as well as the evolving causes of changes.
References
Goren, A., Viljugrein, H., Rivrud, I. M., Jore, S., Bakka, H., Vindenes, Y., & Mysterud, A. (2023). The emergence and shift in seasonality of Lyme borreliosis in Northern Europe. Proceedings of the Royal Society B: Biological Sciences, 290(1993), 20222420. https://doi.org/10.1098/rspb.2022.2420
Hoch, T., Madouasse, A., Jacquot, M., Wongnak, P., Beugnet, F., Bournez, L., . . . Agoulon, A. (2023). Seasonality of host-seeking Ixodes ricinus nymph abundance in relation to climate. bioRxiv, ver.4 peer-reviewed and recommended by Peer Community In Ecology. https://doi.org/10.1101/2022.07.25.501416
Uusitalo, R., Siljander, M., Lindén, A., Sormunen, J. J., Aalto, J., Hendrickx, G., . . . Vapalahti, O. (2022). Predicting habitat suitability for Ixodes ricinus and Ixodes persulcatus ticks in Finland. Parasites & Vectors, 15(1), 310. https://doi.org/10.1186/s13071-022-05410-8
Wongnak, P., Bord, S., Jacquot, M., Agoulon, A., Beugnet, F., Bournez, L., . . . Chalvet-Monfray, K. (2022). Meteorological and climatic variables predict the phenology of Ixodes ricinus nymph activity in France, accounting for habitat heterogeneity. Scientific Reports, 12(1), 7833. https://doi.org/10.1038/s41598-022-11479-z
Reviews: 3
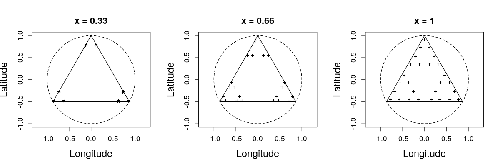
Efficient sampling designs to assess biodiversity spatial autocorrelation : should we go fractal?
Spatial patterns and autocorrelation challenges in ecological conservation
Recommended by Eric Goberville based on reviews by Nigel Yoccoz and Charles J Marsh“Pattern, like beauty, is to some extent in the eye of the beholder” (Grant 1977 in Wiens, 1989)
Evolutionary determinants of reproductive seasonality: a theoretical approach
When does seasonal reproduction evolve?
Recommended by Tim Coulson based on reviews by Francois-Xavier Dechaume-Moncharmont, Nigel Yoccoz and 1 anonymous reviewerHave you ever wondered why some species breed seasonally while others do not? You might think it is all down to lattitude and the harshness of winters but it turns out it is quite a bit more complicated than that. A consequence of this is that climate change may result in the evolution of the degree of seasonal reproduction, with some species perhaps becoming less seasonal and others more so even in the same habitat.
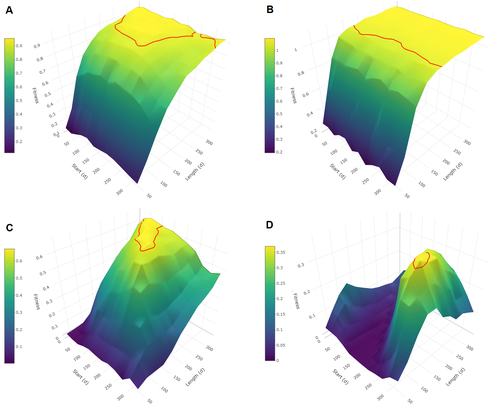
Burtschell et al. (2023) investigated how various factors influence seasonal breeding by building an individual-based model of a baboon population from which they calculated the degree of seasonality for the fittest reproductive strategy. They then altered key aspects of their model to examine how these changes impacted the degree of seasonality in the reproductive strategy. What they found is fascinating.
The degree of seasonality in reproductive strategy is expected to increase with increased seasonality in the environment, decreased food availability, increased energy expenditure, and how predictable resource availability is. Interestingly, neither female cycle length nor extrinsic infant mortality influenced the degree of seasonality in reproduction.
What this means in reality for seasonal species is more challenging to understand. Some environments appear to be becoming more seasonal yet less predictable, and some species appear to be altering their daily energy budgets in response to changing climate in quite complex ways. As with pretty much everything in biology, Burtschell et al.'s work reveals much nuance and complexity, and that predicting how species might alter their reproductive timing is fraught with challenges.
The paper is very well written. With a simpler model it may have proven possible to achieve analytical solutions, but this is a very minor gripe. The reviewers were positive about the paper, and I have little doubt it will be well-cited.
REFERENCES
Burtschell L, Dezeure J, Huchard E, Godelle B (2023) Evolutionary determinants of reproductive seasonality: a theoretical approach. bioRxiv, 2022.08.22.504761, ver. 2 peer-reviewed and recommended by Peer Community in Ecology. https://doi.org/10.1101/2022.08.22.504761
Four decades of phenology in an alpine amphibian: trends, stasis, and climatic drivers
Alpine ecology and their dynamics under climate change
Recommended by Sergio Estay based on reviews by Nigel Yoccoz and 1 anonymous reviewerResearch about the effects of climate change on ecological communities has been abundant in the last decades. In particular, studies about the effects of climate change on mountain ecosystems have been key for understanding and communicating the consequences of this global phenomenon. Alpine regions show higher increases in warming in comparison to low-altitude ecosystems and this trend is likely to continue. This warming has caused reduced snowfall and/or changes in the duration of snow cover. For example, Notarnicola (2020) reported that 78% of the world’s mountain areas have experienced a snow cover decline since 2000. In the same vein, snow cover has decreased by 10% compared with snow coverage in the late 1960s (Walther et al., 2002) and snow cover duration has decreased at a rate of 5 days/decade (Choi et al., 2010). These changes have impacted the dynamics of high-altitude plant and animal populations. Some impacts are changes in the hibernation of animals, the length of the growing season for plants and the soil microbial composition (Chávez et al. 2021).
Lenzi et al. (2023), give us an excellent study using long-term data on alpine amphibian populations. Authors show how climate change has impacted the reproductive phenology of Bufo bufo, especially the breeding season starts 30 days earlier than ~40 years ago. This earlier breeding is associated with the increasing temperatures and reduced snow cover in these alpine ecosystems. However, these changes did not occur in a linear trend but a marked acceleration was observed until mid-1990s with a later stabilization. Authors associated these nonlinear changes with complex interactions between the global trend of seasonal temperatures and site-specific conditions.
Beyond the earlier breeding season, changes in phenology can have important impacts on the long-term viability of alpine populations. Complex interactions could involve positive and negative effects like harder environmental conditions for propagules, faster development of juveniles, or changes in predation pressure. This study opens new research opportunities and questions like the urgent assessment of the global impact of climate change on animal fitness. This study provides key information for the conservation of these populations.
References
Chávez RO, Briceño VF, Lastra JA, Harris-Pascal D, Estay SA (2021) Snow Cover and Snow Persistence Changes in the Mocho-Choshuenco Volcano (Southern Chile) Derived From 35 Years of Landsat Satellite Images. Frontiers in Ecology and Evolution, 9. https://doi.org/10.3389/fevo.2021.643850
Choi G, Robinson DA, Kang S (2010) Changing Northern Hemisphere Snow Seasons. Journal of Climate, 23, 5305–5310. https://doi.org/10.1175/2010JCLI3644.1
Lenzi O, Grossenbacher K, Zumbach S, Lüscher B, Althaus S, Schmocker D, Recher H, Thoma M, Ozgul A, Schmidt BR (2022) Four decades of phenology in an alpine amphibian: trends, stasis, and climatic drivers.bioRxiv, 2022.08.16.503739, ver. 3 peer-reviewed and recommended by Peer Community in Ecology. https://doi.org/10.1101/2022.08.16.503739
Notarnicola C (2020) Hotspots of snow cover changes in global mountain regions over 2000–2018. Remote Sensing of Environment, 243, 111781. https://doi.org/10.1016/j.rse.2020.111781